Viola hirsutula Brainerd
Common names:
Southern Wood Violet
Synonyms:
Viola hirsutula Brainerd, Rhodora 9: 98. 1907 [replacement name for Viola villosa Nutt.]; Viola villosa Nutt., Gen. N. Amer. Pl.: 148. 1818 [not V. villosa Walter (1788)]. TYPE: North Carolina, Tryon, Rich open woodland, 14 Apr 1909, E. Brainerd 64. (NEOTYPE (designated by Ballard et al. Journal of the Botanical Research Institute of Texas 14(2): 222.): GH00067133!, internet image!; ISONEOTYPE: NO0109933, internet image!).
Viola ciliata Willd. ex Schult., Syst. veg. 5: 360. 1819 [this name needs a proposal for rejection]
Viola hirsutula Brainerd f. albicans L.K.Henry, Castanea 18: 46. 1953
Description:
Acaulescent rosulate perennials from thick rhizome, ≤ 16 cm tall; foliage and peduncles dark green, upper surface of leaf blades light gray- or silvery-green with dark green or reddish-purple veins, lower surface of blades and peduncles often purple tinged, all glabrous except for uniformly distributed conspicuous spreading or erect hairs on upper surface of leaf blades; stipules free, irregularly glandular-fimbriate; leaves spreading to prostrate (commonly lying on substrate during summer fruit), leaf blades undivided, largest ≤ 55 × 45 mm, ovate to suborbicular, base cordate, margins crenate to serrate, eciliate, apex acute to rounded; chasmogamous peduncle held above the leaves; chasmogamous flower ≤ 18 mm; calyx glabrous, eciliate; lowest sepals ovate (uncommonly ovate-lanceolate), obtuse to rounded; auricles short and entire, not elongating in fruit; corolla blue to purple or reddish-purple, throat white; spur short-globose; lateral petals densely bearded with filiform to narrowly linear hairs, spurred petal densely bearded; chasmogamous capsule green; cleistogamous flowers produced after chasmogamous, peduncle initially prostrate but arching just before capsule dehiscence and shorter than petioles; cleistogamous capsule 6.5–12 mm, green drying tan with purple spots or blotches, glabrous; seeds 1.5–2.1 × 1.1–1.4 mm, very light brown to orange-brown, unspotted or with weak to prominent brown streaks and spots; 2n=54.
Similar species:
This species is distinctive in its spreading to prostrate leaves, the upper surface bicolorous ("variegated") with the silvery-green lamina divided by deep green or red-purple veins and beset with erect hispid hairs. On the basis of the relatively short broad variegated leaves alone, it could be misidentified as V. walteri (which is mat-forming by node-rooting stolons) or the acaulescent southern V. villosa that does not enter our range, but the foliage and peduncle are glabrous except for the small hispid hairs on the upper leaf blade surface.
Ecology:
Sandy soils on ridgetops, slopes of dry or dry-mesic oak and oak-pine or conifer forests, moist slopes or bottomlands, and clearings in former natural wooded habitats.
Distribution:
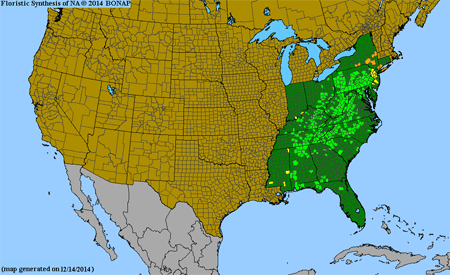
Primarily Appalachian Mountain highlands and associated uplands but extending southward, CT and NY to OH, south to the panhandle of FL, MS, c. KY and se. IN.
Rarity:
State listed in CT, IN, NJ, and NY.
Phenology:
Chasmogamous flower February–May, chasmogamous fruit May, cleistogamous fruit May–August.
Affinities:
This species belongs to the Acaulescent Blue Violet lineage, sect. Nosphinium W.Becker, subsect. Boreali-Americanae (W.Becker) Gil-ad, in the Sororia species group.
Hybrids:
Hybridizes with V. affinis (Brainerd 1906b, 1924, House 1906b, 1924, Dowell 1910), V. communis [or possibly V. "sororia glabrous" or V. "sororia hirsutula-like"] (House 1906b, Brainerd 1907a, 1907b, 1924, Henry 1953a), V. emarginata sensu stricto (House 1906b, Malte & Macoun 1915, House 1924), V. fimbriatula (Dowell 1910, Brainerd 1924, Henry 1953a), V. palmata var. triloba (Brainerd 1906b, 1912, 1924, House 1924), V. sagittata (Henry 1953a), V. sororia sensu stricto (Brainerd 1924, Henry 1953a), V. stoneana (House 1906b, Brainerd 1912, 1924), and V. subsinuata sensu stricto (Brainerd 1906b, 1912, 1924). Brainerd reported that these exhibit intermediate or recombinant characteristics of foliage, chasmogamous flowers, cleistogamous capsules and seeds (where these did not abort). None of the hybrids reproduce by chasmogamous means. Some hybrids produce abortive cleistogamous flowers or capsules, while the remaining hybrids produce normal cleistogamous capsules but with substantial to dramatic reduction in the proportion of viable seeds.
Comments:
Brainerd cited no type in the protologue, but following publication of the name he distributed specimens under his No. 64. No earlier collections named by him have been found at. McKinney (1992) incorrectly annotated the GH00067133 sheet as the holotype but later stated the type as "unknown". JSTOR Global Plants includes the GH sheet and a NO sheet of Brainerd 64, but the collections post-date Brainerd’s publication and are therefore not original material. In the absence of original material from which to select a lectotype, the GH and NO sheets were designated as neotype and isoneotype, respectively, by Ballard et al. (2020).
This species has been recognized by Brainerd (1924), Brainerd Baird (1942), Fernald (1950), Henry (1953a), Alexander (1963), Russell (1965), Strausbaugh and Core (1978), McKinney (1992), Ballard (2000), McKinney and Russell (2002), Haines et al. (2011), Weakley et al. (2012), and Little and McKinney (2015). In their extensive merger, Gleason and Cronquist (1991) synonymized it under V. villosa. Gil-ad (1995, 1997, 1998) conducted field studies and examined three samples for micromorphological traits; seeds from Brainerd's original material were not available. He discovered no unique micromorphological features, with some suggestive of V. affinis, V. missouriensis or V. sororia. He argued that the taxon possessed no unique macromorphological features, noting that the foliage coloration could be attributed to V. nephrophylla, the pubescence pattern is similar to that of V. cucullata and other species, and that reproductive characters resembled those of other species. He pointed out the variable habitats occupied by the species as final evidence that this species is of hybrid origin and should be abandoned. His micromorphological sampling, as cited in the dissertation, is both sparse and problematic; one study sample was collected from Taberville Prairie in Missouri, nearly 400 miles west of the confirmed western limit of this species in Calloway and Henderson Counties of Kentucky. Ballard (2013) made no mention of the presence of V. hirsutula in Missouri. I have examined mature cleistogamous seeds from Alabama (Brainerd 65), Indiana (Deam 20567), Maryland (House 1002), North Carolina (Ballard 15-012) and elsewhere and found them quite uniform in dimensions and color pattern. Herbarium specimens from throughout the primarily Appalachian range of the species, when not adulterated by frequent hybridization, are also highly uniform in traits of foliage, chasmogamous flowers, and cleistogamous fruits. The dry to dry-mesic woodland habitat, often in the vicinity of V. palmata and V. sororia sensu stricto with which it commonly hybridizes, is no more variable than any other woodland violet. The suite of macromorphological features that distinguishes this species is unique in the lineage, and its woodland ecological niche is different from most other species (although it makes contact with a few other species regularly). Under the Unified Species Concept, the uniformity of morphological traits within and across populations over its range, the suite of consistent vegetative and reproductive differences separating it and other species, and reduced fertility of hybrids with other species, provide compelling evidence for recognizing V. hirsutula as a distinct species. It is distinctive vegetatively, with foliage strictly glabrous except for the conspicuous spreading to erect hairs on the upper surface of the leaf blades, the bicolorous upper surface with gray- to silvery-green lamina between contrasting dark green or red-purple primary veins, and the tendency of the leaves (especially in fruit) to be horizontally oriented and eventually lie flat on the substrate. Hybrids express the striking bicolorous color pattern and the conspicuous spreading to erect hairs of the upper leaf blade surface; the frequent hybrid with V. palmata is particularly beautiful.
Literature Cited:
Alexander, E. J. 1963. Violaceae. In Gleason, H. A., The new Britton and Brown illustrated flora of the northeastern United States and adjacent Canada. Hafner Publishing Co., Inc., New York, NY. 552-567.
Ballard Jr., H. E. 2000. Violaceae. In Rhoads, A. (ed.). Flora of Pennsylvania. University of Pennsylvania Press, Philadelphia, PA. 700-710.
Ballard Jr., H. E. 2013. Violaceae. Yatskievych, G., Flora of Missouri, 1218-1243. Missouri Botanical Garden Press, St. Louis, MO.
Ballard Jr., H. E., R. N. Burwell, and S. L. Lockhart. 2020. Violaceae: Typifications and clarifications of names. In Weakley, A. S., D. B. Poindexter, H. C. Medford, B. A. Sorrie, C. A. McCormick, E. L. Bridges, S. L. Orzell, K. A. Bradley, H. E. Ballard, Jr., R. N. Burwell, S. L. Lockhart, and A. R. Franck. Studies in the vascular flora of the southeastern United States. VI. Journal of the Botanical Research Institute of Texas 14(2): 217-229.
Brainerd, E. 1906b. Hybridism in the genus Viola,-II. Rhodora 8: 6–10.
Brainerd, E. 1907a. Mendel’s law of dominance in the hybrids of Viola. Rhodora 9: 211–216.
Brainerd, E. 1907b. The behavior of seedlings of certain violet hybrids. Science 25: 940-944.
Brainerd, E. 1912. Violet hybrids between species of the palmata group. Bulletin of the Torrey Botanical Club 39: 85–97.
Brainerd, E. 1921b. Violets of North America. Vermont Agricultural Experiment Station Bulletin 224: 1–172.
Brainerd, E. 1924. The natural violet hybrids of North America. Vermont Agricultural Experiment Station Bulletin 239.
Brainerd Baird, V. 1942. Wild violets of North America. University of California Press, Berkeley, CA.
Dowell, P. 1910. The violets of Staten Island. Bull. Torr. Bot. Club 37: 163-179, pl. 11-18.
Fernald, M. L. 1950. Violaceae. In Gray’s Manual of Botany, 8th ed. American Book Company, New York, NY. 1022-1042.
Gil-ad, N. L. 1995. Systematics and evolution of Viola L. subsection Boreali-Americanae (W. Becker) Brizicky. Ph.D. dissertation. University of Michigan, Ann Arbor, MI.
Gil-ad, N. L. 1997. Systematics of Viola subsection Boreali-Americanae. Boissiera 53: 1–130.
Gil-ad, N. L. 1998. The micromorphologies of seed coats and petal trichomes of the taxa of Viola subsect. Boreali-Americanae (Violaceae) and their utility in discerning orthospecies from hybrids. Brittonia 50: 91–121.
Gleason, H. A. and A. Cronquist. 1991. Violaceae. In Manual of vascular plants of northeastern United States and adjacent Canada, 2nd ed. New York Botanical Garden, Bronx, NY. 157-163.
Haines, A., E. Farnsworth, and G. Morrison. 2011. Violaceae. In Flora Novae Angliae. Yale University Press, New Haven, CT. 873-886.
Henry, L. K. 1953a. The Violaceae in western Pennsylvania. Castanea 18(2): 37-59.
House, H. D. 1906b. The violets and violet hybrids of the District of Columbia and vicinity. Rhodora 8: 117-122, plates 71-72.
House, H. D. 1924. Annotated list of the ferns and flowering plants of New York state. Family 83 Violaceae. New York State Museum Bulletin 254: 499-512.
Little, R. J., and L. E. McKinney. 2015. Violaceae. In Flora of North America: Cucurbitaceae to Droseraceae, 106. Oxford University Press, New York, NY.
Malte, M. O. and J. Macoun. 1915. Ottawa Naturalist 28: 145-150, 161-168.
McKinney, L. E. 1992. A taxonomic revision of the acaulescent blue violets (Viola) of North America. Sida, Botanical Miscellany 7: 1–60.
McKinney, L. E., and N. H. Russell. 2002. Violaceae of the southeastern United States. Castanea 67: 369–379.
Russell, N. H. 1965. Violets (Viola) of the central and eastern United States: An introductory survey. Sida 2: 1-113
Strausbaugh, P. D. and E. L. Core. 1978. Violaceae. In Flora of West Virginia, 2nd ed. Seneca Books, Inc., Morgantown, WV. 644-658.
Weakley, A. S., J. C. Ludwig, and J. F. Townsend. 2012. Violaceae. In Flora of Virginia. BRIT Press, Fort Worth, TX. 963-975.

Chasmogamous flowering habit by Bruce Sorrie

Leaves by Andrew Gibson, "Buckeye Botanist" website

Chasmogamous flower front view by Bruce Sorrie

Seeds from herbarium specimen: IN, C. C. Deam 20567 (NY)

Seeds from herbarium specimen: Transplanted from NC, Montgomery Co., Mocassin Creek Road, H. E. Ballard 15-012X (BHO)
Map by the Biota of North America Program