Viola latiuscula Greene
Common names:
Broad-leaved Wood Violet
Synonyms:
Viola latiuscula Greene, Pittonia 5: 93. 1902. Viola latiuscula Greene, Pittonia 5: 93. 1902. TYPE: "This very satisfactory new violet is from Twin Mountains, West Rutland, Vermont, and was collected May 24 and July 15 of 1902, by Mr. W. W. Eggleston, who writes that it grows in open shady well drained soil." TYPE: Vermont, Twin Mountains, West Rutland, 24 May, W. W. Eggleston 2648 (LECTOTYPE (designated by Ballard et al. 2020. Journal of the Botanical Research Institute of Texas 14(2): 223): NDG32742[a]!*; ISOLECTOTYPES: CM1457[a][barcode, accession # is CM107917]*, CM1458[a][barcode, accession # is CM107918]*, GH00067135[a]!*, ILL00006622[a]*, MIN1002774[a]*, MU000000218[a]*, NY00097537[a]!*, NY00097538![a]*, NY00097539[a]!*, PH00029251[a]*, PH00029252[a]*, PH00029253[a]*, RSA0006446[a]*, S-G-6375[a]*, UVMVT080304!*, UVMVT083115!*, and YU069971[a], *internet image seen)).
Description:
Acaulescent rosulate perennials from thick rhizome, ≤ 28 cm tall; foliage and peduncles green, lower surface of leaf blades commonly purple-tinged, glabrous except for papillate or puberulent summit of petioles and scattered small hairs on the upper surface of leaf blades; stipules free, irregularly glandular-fimbriate; leaves ascending, leaf blades undivided, largest ≤ 99 × 145 mm, leaf blades during chasmogamous flower slightly longer than broad or as broad as long and deltate-triangular, apex acute to short-acuminate, during fruit becoming much broader than long, deltate-reniform, apex abruptly acute or obtuse, base truncate to shallowly cordate, margins crenate, eciliate; chasmogamous peduncle usually held above the leaves; chasmogamous flower ≤ 16 mm; calyx glabrous, eciliate; lowest sepals oblong-lanceolate to ovate-lanceolate, acute or acutish; auricles short and entire, not or scarcely elongating in fruit; corolla blue to purple, throat white; spur short-globose; lateral petals densely bearded with narrowly linear to slightly clavate hairs, spurred petal densely bearded; chasmogamous capsule green; cleistogamous flowers produced after chasmogamous, peduncle initially prostrate but arching just before capsule dehiscence, much shorter than petioles; cleistogamous capsule 6–11 mm, yellow-green drying tan with usually weak purple spots or blotches, glabrous; seeds 1.3–2.0 × 1.1–1.3 mm, purplish-black, with very weak darker blotches, spots or streaks; 2n=54.
Similar species:
This species is similar to a few Borealiamericanae taxa with glabrous foliage and leaves nearly as broad as or broader than long. In chasmogamous flower it differs from V. communis, V. domestica, V. sororia [glabrous variant] and V. sororia [hirsutuloides variant] in its densely bearded spurred petal, from V. nephrophylla in its acute sepals, and from V. pratincola and V. retusa in its short rounded auricles. In cleistogamous fruit it is distinguished from other species by its broadly deltate-reniform leaf blades with truncate to subcordate base and obtuse to abruptly broadly acute apex, from V. communis, V. domestica, V. pratincola and V. retusa in its spotted or blotched cleistogamous capsule on a short prostrate peduncle, from V. affinis and V. missouriensis by its purplish black seeds and additionally from the latter in its acute eciliate sepals, and from the V. sororia variants by its purplish-black weakly marked seeds.
Ecology:
Dry sandy or gravelly soils of upland deciduous or mixed forests, and dry ledges.
Distribution:
Northern Appalachian Mountains and e. Great Lakes woodlands, VT and NH west and south to sw. NY and s.-c. PA.
Rarity:
None.
Phenology:
Chasmogamous flower May–June, chasmogamous fruit June–July, cleistogamous fruit June–September.
Affinities:
This species belongs to the Acaulescent Blue Violet lineage, sect. Nosphinium W.Becker, subsect. Boreali-Americanae (W.Becker) Gil-ad, in the Sororia species group.
Hybrids:
Hybridizes with V. fimbriatula (Brainerd 1924, House 1924), V. palmata var. triloba (Brainerd 1912, 1924, House 1924) and V. sororia sensu stricto (Brainerd 1924, House 1924). Brainerd reported that these exhibit intermediate or recombinant characteristics of foliage, chasmogamous flowers, cleistogamous capsules and seeds (where these did not abort). Both hybrids fail to reproduce by chasmogamous flowers. The first hybrid reportedly produces very few viable cleistogamous seeds.
Comments:
Greene provided explicit information in his protologue, stating "This very satisfactory new violet is from Twin Mountains, West Rutland, Vermont, and was collected May 24 and July 15 of 1902, by Mr. W.W. Eggleston, who writes that it grows in open shady well drained soil." Because he cited two separate collections, did not indicate which sheets may be types, and mentioned no herbarium, a lectotype needed to be designated. Several sheets are in the JSTOR Global Plants database, and sheets vary as to collection date and collector number. All specimens include 24 May 1902 and 14 Jul 1902 on printed labels, while only the NDG32742 sheet also includes 15 July 1902 on its handwritten label. Furthermore, the NDG sheet has "Viola latiuscula, Green, type" written in Greene’s hand across the top of the label. McKinney incorrectly designated the May 1902 collection of NY00097537 as holotype. The three flowering plants from the 24 May 1902 collection (Eggleston 2648) on the NDG32742 sheet (denoted by "[a]") were selected by Ballard et al. (2020) as the lectotype. Other 24 May specimens of Eggleston 2648 were treated as isolectotypes. The sterile or fruiting specimens collected on 15 July 1902 (Eggleston 2867) on the NDG32742 sheet (denoted by “[b]” are a syntype, while specimens with the 14 July 1902 collection date are considered original material.
Brainerd (1921b), Brainerd Baird (1942), Fernald (1950), Henry (1953a), and Alexander (1963) accepted this species; Fernald included Virginia in its range, but his records from marl pits, rich woods and brushy clearings in the Coastal Plain counties of Dinwiddie, Greensville and Sussex Counties (Fernald 1941) do not match the habitats of the present species and the localities are well out of range for a northern montane species. Fernald's Virginia records are rejected here as probable hybrids of other species. Alexander included New Jersey, and specimens need examination for confirmation. Scoggan (1978) mentioned specimens from three Ontario counties near Niagara Falls matching this species that needed verification; however, a number of specimens have been confirmed on the New York state side to corroborate its possible occurrence in extreme southeastern Ontario. Gleason and Cronquist (1991), Ballard (2000), Weakley et al. (2012), and Little and McKinney (2015) included it in V. sororia; while McKinney (1992) and McKinney and Russell (2002) included it in V. sororia var. sororia. Haines et al. included it partly in V. affinis and partly in V. sororia. Russell (1965) made a mystifying and inaccurate reference to cut leaf blades and southern geographic distribution, indicating that he confused this with another taxon (perhaps V. chalcosperma), but he noted that the granular or pubescent petiole summit mentioned by Fernald (1950) as distinctive was present in occasional Appalachian specimens of V. affinis and excluded the taxon. Gil-ad (1995, 1997) was unable to study mature seeds but enumerated the features given by others, including Greene, and dismissed the taxon due to the lack of unique "suite of characters" and postulated that it is likely a hybrid involving V. sororia, and V. affinis or V. cucullata. Examination of type material and quite a few other herbarium specimens matching the description has confirmed a consistent suite of diagnostic features: densely bearded spurred petal, essentially glabrous foliage with granular or puberulent petiole summit, frequent small scattered subappressed hairs on the upper surface of the leaf blades, short and very broadly deltate-reniform summer leaf blades with a shallowly cordate to subtruncate base and obtuse or (more commonly) abruptly acute apex, a common purple tinge on the lower surface of the leaf blades, ovate-lanceolate to ovate broadly acute to obtuse lowest sepals, and purple-black seeds with very weak darker blotches, streaks or spots, often with a relatively long thick caruncle. The first largest leaf blade during chasmogamous flower is often somewhat longer than broad, while all subsequent leaf blades are broadly ovate, broadening in fruit to a distinctive and remarkably short broad deltate-reniform outline with shallowly cordate to truncate base and obtuse to abruptly acute apex. This violet also expresses a preference for dry sandy woodland soils and dry ledges that is quite divergent from other species in the region. It is accepted here as a distinct species which expresses a unique suite of morphological traits and a xeric woodland niche. Russell's specimens of "otherwise affinis" in the Appalachian Mountains deserve scrutiny to determine whether he misidentified additional specimens of this species. This narrow regional endemic is rather poorly understood.
Literature Cited:
Alexander, E. J. 1963. Violaceae. In Gleason, H. A., The new Britton and Brown illustrated flora of the northeastern United States and adjacent Canada. Hafner Publishing Co., Inc., New York, NY. 552-567.
Ballard Jr., H. E. 2000. Violaceae. In Rhoads, A. (ed.). Flora of Pennsylvania. University of Pennsylvania Press, Philadelphia, PA. 700-710.
Ballard Jr., H. E., R. N. Burwell, and S. L. Lockhart. 2020. Violaceae: Typifications and clarifications of names. In Weakley, A. S., D. B. Poindexter, H. C. Medford, B. A. Sorrie, C. A. McCormick, E. L. Bridges, S. L. Orzell, K. A. Bradley, H. E. Ballard, Jr., R. N. Burwell, S. L. Lockhart, and A. R. Franck. Studies in the vascular flora of the southeastern United States. VI. Journal of the Botanical Research Institute of Texas 14(2): 217-229.
Brainerd, E. 1912. Violet hybrids between species of the Palmata group. Bulletin of the Torrey Botanical Club 39: 85-97.
Brainerd, E. 1921b. Violets of North America. Vermont Agricultural Experiment Station Bulletin 224: 1–172.
Brainerd, E. 1924. The natural violet hybrids of North America. Vermont Agricultural Experiment Station Bulletin 239: 1-205.
Brainerd Baird, V. 1942. Wild violets of North America. University of California Press, Berkeley, CA.
Dowell, P. 1910. The violets of Staten Island. Bull. Torr. Bot. Club 37: 163-179, pl. 11-18.
Fernald, M. L. 1941. Contributions from the Gray Herbarium of Harvard University–No. CXXXIX: Another century of additions to the flora of Virginia. Rhodora 43: 485-553, 559-630, 635-657.
Fernald, M. L. 1950. Violaceae. In Gray’s Manual of Botany, 8th ed. American Book Company, New York, NY. 1022-1042.
Gil-ad, N. L. 1995. Systematics and evolution of Viola L. subsection Boreali-Americanae (W. Becker) Brizicky. Ph.D. dissertation. University of Michigan, Ann Arbor, MI.
Gil-ad, N. L. 1997. Systematics of Viola subsection Boreali-Americanae. Boissiera 53: 1–130.
Gleason, H. A. and A. Cronquist. 1991. Violaceae. In Manual of vascular plants of northeastern United States and adjacent Canada, 2nd ed. New York Botanical Garden, Bronx, NY. 157-163.
Haines, A., E. Farnsworth, and G. Morrison. 2011. Violaceae. In Flora Novae Angliae. Yale University Press, New Haven, CT. 873-886.
Henry, L. K. 1953a. The Violaceae in western Pennsylvania. Castanea 18(2): 37-59.
House, H. D. 1924. Annotated list of the ferns and flowering plants of New York state. Family 83 Violaceae. New York State Museum Bulletin 254: 499-512.
Little, R. J., and L. E. McKinney. 2015. Violaceae. In Flora of North America: Cucurbitaceae to Droseraceae, 106. Oxford University Press, New York, NY.
McKinney, L. E. 1992. A taxonomic revision of the acaulescent blue violets (Viola) of North America. Sida, Botanical Miscellany 7: 1–60.
McKinney, L. E., and N. H. Russell. 2002. Violaceae of the southeastern United States. Castanea 67: 369–379.
Russell, N. H. 1965. Violets (Viola) of the central and eastern United States: An introductory survey. Sida 2: 1-113
Scoggan, H. J. 1978. Violaceae. In Flora of Canada, Part 3–Dicotyledoneae (Saururaceae to Violaceae). National Museums of Canada. Ottawa, Canada. 1103-1115.
Weakley, A. S., J. C. Ludwig, and J. F. Townsend. 2012. Violaceae. In Flora of Virginia. BRIT Press, Fort Worth, TX. 963-975.

Chasmogamous flowering habit from specimen: VT, Twin Mountains, West Rutland, 24 May 1902, W. W. Eggleston 2648 (UVMVT083115, syntype of Viola latiuscula)

Chasmogamous flowering habit from herbarium specimen: VT, Salisbury, Dry open woodland, 29 May 1910, E. Brainerd 75 (NY)

Cleistogamous fruiting habit from herbarium specimen: VT, Twin Mountains, West Rutland, 14 Jul 1902, W. W. Eggleston 2867 (UVMVT080304, syntype of Viola latiuscula Greene)

Cleistogamous fruiting habit from herbarium specimen: VT, Ft. Ethan Allen, Dry sandy copse, 11 Sep 1903, E. Brainerd 220 (NY)

Leaves from herbarium specimen: PA, Monroe Co., Buck Hill Falls, 10-14 Jul 1903, W. Stone 5480 (PH00226717)

Chasmogamous flower front view from specimen: VT, Twin Mountains, West Rutland, 24 May 1902, W. W. Eggleston 2648 (UVMVT083115, syntype of Viola latiuscula)

Chasmogamous flower profile view from herbarium specimen: VT, Salisbury, Dry open woodland, 29 May 1910, E. Brainerd 75 (NY)

Cleistogamous fruit with immature seeds from herbarium specimen: VT, Twin Mountains, West Rutland, 14 Jul 1902, W. W. Eggleston 2867 (UVMVT080304, syntype of Viola latiuscula Greene)

Cleistogamous fruits from herbarium specimen: VT, Ft. Ethan Allen, Dry sandy copse, 11 Sep 1903, E. Brainerd 220 (NY)

Seeds (pale ones immature) from herbarium specimen: VT, Salisbury, Lake Dumrose, Recent clearing, 21 Aug 1903, E. Brainerd s.n. (YU244844)

Seeds (pale ones immature) from herbarium specimen: Transplanted from NY, Falconer, May 1910 [Slavin donor], 3 Aug 1910, E. Brainerd 76 (NY)
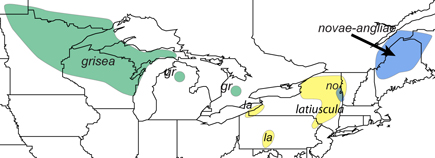
Map of northern members of the Sororia species group by Harvey Ballard