
Basics

 Types of Energy
Types of Energy
 Types of Energy
Types of Energy

 Types of Energy
Types of Energy|
Because energy, the ability to do work, appears in such a wide variety of
physical systems, there are many different kinds of energy. The division of
energy into different types is somewhat arbitrary, but will prove convenient to recognize two very broad categories. Kinetic energy is energy associated with moving objects, while stored or potential energy is energy waiting to be released. Our intuition tells us that two factors govern the amount of objects' kinetic energy.
First, heavy objects have more energy than light ones: a bowling ball going with 10 meters per second ( a very fast sprint) carries a lot more kinetic energy than a golf ball going
at the same velocity. Kinetic energy is proportional to mass: double the mass, double the kinetic energy.
In words:
In equation form:
In symbols: |
Example
Reasoning: = 1/2 * 4 kg * 100 m2/s2 = 200 kg m2/s2 = 200 J For the baseball traveling at 50 m/s: = 1/2 * 0.25 (kg) * 2500 m2/s2 = 312.5 kg m2/s2 = 312.5 J |
|
The kind of energy, that could result in the exertion of a force over a distance, but is not doing so, is called Potential Energy. An object that has been lifted above the surface of the earth possesses an amount of gravitational potential energy exactly equal to the total amount of work you would have to do to lift it from the ground for the present position.
In words:
In equation form: where g is the acceleration due to gravity at the earth's surface.
In symbols: |
|
 Potential Energy: Potential Energy:
Stored energy Work = weight h
= m g h
Drop box : gravity --> acceleration
At floor level : maximum speed
 Potential Energy : m g h Potential Energy : m g h
 Kinetic Energy : 1/2 m v2 Kinetic Energy : 1/2 m v2
 Conservation of Energy Conservation of Energy
| ||
|
During the swing of a pendulum potential energy is converted to kinetic energy and vice versa. The potential energy is maximum at the maximum swing of the pendulum, the kinetic energy is maximum when the pendulum is at its lowest point. |
 Temperature
Temperature |
One example of stored energy: internal energy ==> vibration of molecules
 Thermal Energy Thermal Energy
Properties of a substance which depends on the temperature:
|
|
How do we measure temperature? |
|
Since we really don't yet know what temperature is, we
have to measure the consequences of it. It is empirically known that when
many object get hotter, that they expand. This expansion can be used as
a measure for the temperature. Here is one type of thermometers:
 |
|
The fluid in the reservoir is in thermal contact with
the environment. Heat is exchanged until the
temperature of the reservoir and the environment is the equal. The level
of the fluid is a measure for the temperature.
Problem: Not very precise since the thermal expansion rate depends on the fluid used. It is limited to a smaller range of temperatures.
|
|
Temperature Scale:
|
The pressure measured by the level h in the gas thermometer
is determined at the steam point and the ice point of water. The steam
point is defined as 1000
Celsius (C) and the ice point is defined as 00
Celsius (C). Temperatures between are determined by measuring the pressure
with the gas thermometer and then interpolating linearly between the boiling
and the freezing point.
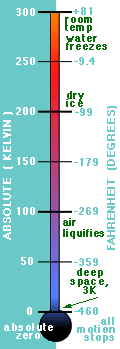
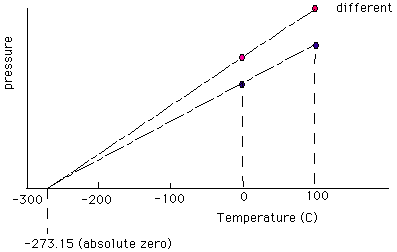 All motion is frozen at this temperature. This temperature is chosen as the zero level of the Kelvin scale. The relation between the Kelvin scale T and the Celsius scale Tc therefore reads |
|
Another scale (used mostly in the US) is the Fahrenheit scale. The steam point is defined as 2120F and the ice-point is defined as 320F. Since the difference of 1800F corresponds to 1000C, the conversion between Fahrenheit and Celsius is |
|
A more precise fix point as a reference for the temperature is the triple point of water. At this point, water, water vapor and ice coexist. This point is at 0.010C = 273.16K. |
|
SI Defintion of 1 Kelvin:
Characteristic Temperatures:
EXAMPLE 1 What is the temperature 15F in Celcius and Kelvin? Most European elementary schools cancel classes when the temperature exceeds 30 degrees Celsius. What is this temperature in Fahrenheit?
Watch the Journey to Absolute Zero |

Ch. Elster