 Light Sources
Light Sources
All hot objects emit light.
Examples:
- Car in the sun --> `heat waves" = infrared (IR)
- Burnger on a stove --> infrared and red light
- Light bulb filament --> all visible light
- sun --> infrared and visible and ultraviolet (UV)
 Black Body Radiation
Black Body Radiation
A body that absorbs all radiation that falls on it is called
black body.
 Cavity with a small entrance hole
Cavity with a small entrance hole
Energy entering a small hole in a chamber rattles around until it is
absorbed. In reverse, the aperture in a heated enclosure appears as
a blackbody source.
When a black body is
heated, it starts to glow and
a characteristic black body spectrum
is emitted.
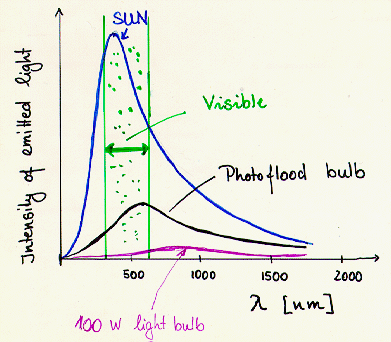
A black body spectrum
has the following properties:
- More
energy is emitted from hot objects than from cool ones.
- The peak emission occurs at
higher
frequencies for hot
objects
and at lower frequencies for
cooler objects.
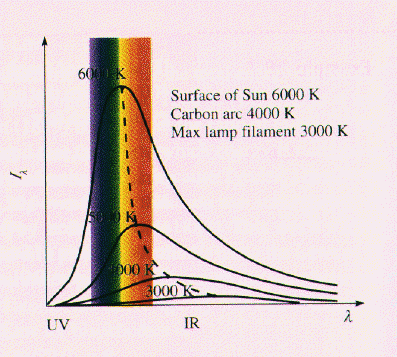 The amount of radiant
energy emitted by a hot object at various wavelengths. Each curve peaks
at a point where
The amount of radiant
energy emitted by a hot object at various wavelengths. Each curve peaks
at a point where 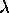 T = constant, which is the Wien Displacement Law.
T = constant, which is the Wien Displacement Law.
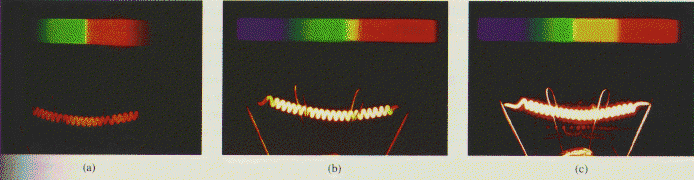
A hot filament and the spectra it emits. As the temperature rises form
(a) to (c), the corresponding emission curves shift, as shown in the
figure above. The peaks of the curves moe toward the yellow, and the bue and of the spectrum increases in intensity as well. The result is that the color
of the filament shifts from cherry red to white hot.
 Incandescent Light
Incandescent Light
is created by the glow of hot objects.
Incandescent electric light consists of hot, glowing
filaments (tungsten filament).
 Light as Particle
Light as Particle
|
| Important Terms related to Atoms |
| Element
| A chemical substance that cannot be broken down further |
| Atom
| The smallest particle that retains its chemical identity |
| Molcule
| Any collection of two or more atoms bound together |
| Electron
| An atomic particle with negative charge and small mass |
| Nucleus
| The small, massive central part of an atom |
| Proton
| Positively charged nuclear particle |
| Neutron
| Electrically neutral nuclear particle |
| Ion
| An electrically charged atom |
 The Bohr Atom
The Bohr Atom
An atom
consists of a positively charged
nucleus and negatively charged
electrons, so that the atom itself is electrically neutral.
(Model of the atom according to Rutherford)
The idea is that the electron can exist at a distance r1
from the nucleus, or at a distance r2 from the
nucleus, or at a distance r3 from the nucleus and so on.
As long as the electron remains at one of those distances, its energy
is fixed. The electron cannot ever, at any time, be in orbit any
place between these allowed distances.
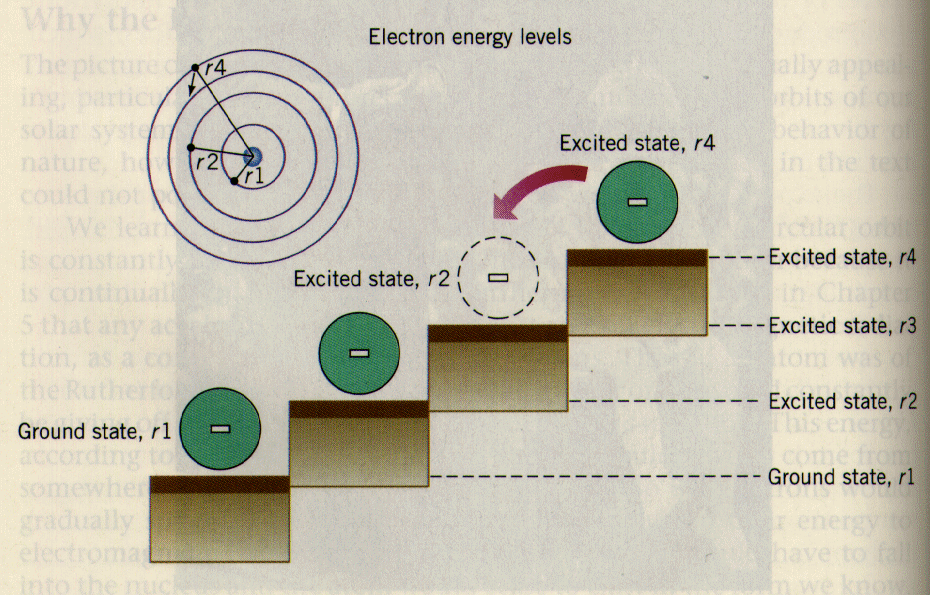
An electron in an atom can be in any of a number of allowed orbits, and
each allowed orbit is at a different energy. One can see this by noting
that one would have to exert a force over a distance to move one
electron from one allowed orbit to another, just as muscles have to exert
a force to kick up a ball a flight of stairs. The allowed energy levels
of an atom occur as a series of steps. The electron in the lowest
level is called the ground state,
while all energy levels above the ground state are called
excited states.
In (a) the electron of a hydrogen atom is in its ground state.
Assume that an electron is in an excited state as shown in (c).
The electron can move to the lowest state, but if it does, something must
happen to the extra energy. The energy that is left over when the
electrically charged electron moves from an upper state to a lower
state is emitted by the atom in form of a single packet of
electromagnetic radiation, a particle-like unit called a
photon. Every time
an electron jumps from a higher to a lower level, a photon moves away
at the speed of light. In this sense, light behaves like a particle,
though it behaves at the same time as a wave.
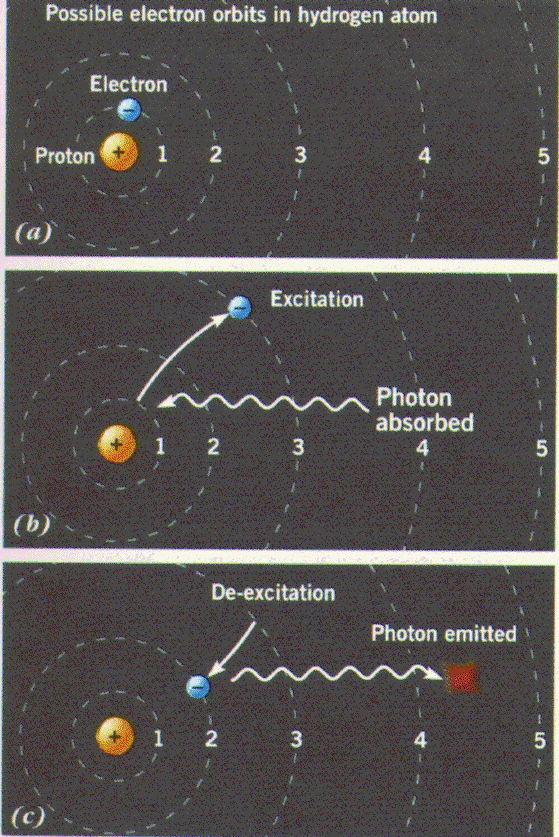
Electrons may jump between the energy levels shown in (a) and
in the process absorb
(b) or emit (c)
energy in form of a photon. This process is called a
quantum leap or
quantum jump and is
fundamental in nature at the atomic scale.
 Sequence of events in (b):
Sequence of events in (b):
- electron in ground state level
- atom absorbs a photon
- electon jumps to an excited energy level
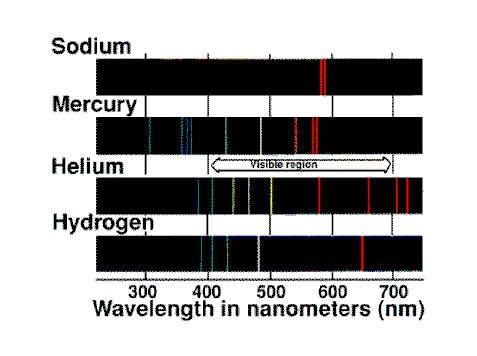
This process is responsible for an absorption spectum.
For example the white light from the sun shows dark absorption
lines due to the cooler gases which surround the sun. The presence of
sodium in these gases is indicated by two dark lines in the yellow part
of the sprectrum. These dark lines are exactly at the same frequencies
where the two bright yellow lines occur in the emission spectrum of
sodium.
 Sequence of events in (c):
Sequence of events in (c):
- electron in excited energy level
- transition to lower energy level
- emission of photons
- photon: quantum of light with a distinct frequency
This process is responsible for an emission
spectrum, which
consists of distinct lines
of a specific color (frequency).
The processes leading to spefic emission lines are shown here:
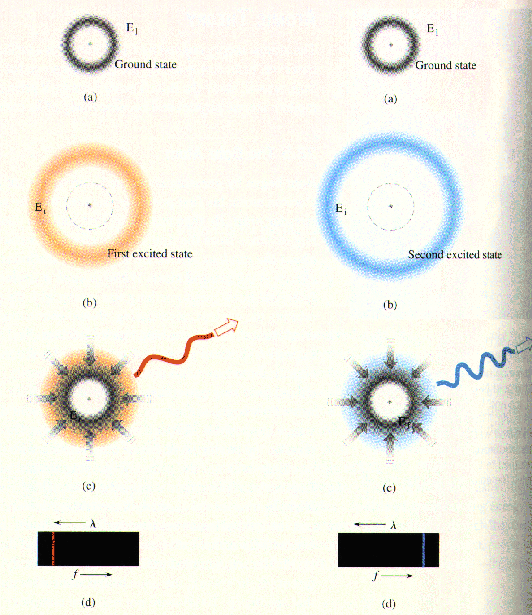
The left panel shows that an atom in the first excited state drops
back to the ground state with the emission of a long-wavelength, low energy
photon. The emission shows up as a long-wavelength, low-frequency spectral
line.
The right panel shows an atom in the second excited state that drops back
to the ground state with the emission of a short-wavelength, high energy
photon.


 Light Sources
Light Sources  Black Body Radiation
Black Body Radiation 
 Incandescent Light
Incandescent Light Light as Particle
Light as Particle
 Cavity with a small entrance hole
Cavity with a small entrance hole
 The amount of radiant
energy emitted by a hot object at various wavelengths. Each curve peaks
at a point where
The amount of radiant
energy emitted by a hot object at various wavelengths. Each curve peaks
at a point where 



