|
 Fluorescence Fluorescence
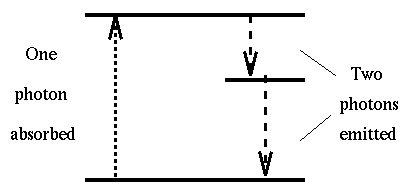
When an atom is excited from one energy state to a higher one by the
aborption of a photon, it may return to the lower level in a series of two
(or more) jumps if there is an energy level in between. The photons emitted
will consequently have lower energy and frequency than the absorbed
photon. When the absorbed photon is in the UV and the emitted photons
are in hte visible region of the spectrum, this phonomenon is
called fluorescence.
Fluorescent objects (phosphors) absorb ultraviolet light
and produce visible light.
Fluorescent lightbulbs
work in a two-step process. The applied voltage accelerates electrons
that strike atoms of the gas in the tube and cause them to be excited.
When the excited atoms jump down to their normal levels, they emit
UV photons which strike a fluorescent coating on the inside of the
tube. The light we see is a result of this material fluorescing in response
to the UV light striking it.
Watch:
How does Flourescence
Work?
Fantastic Flourescence
LIGHT SOURCES
and their
| Approximate Efficiency |
| Fire (torches)
| 0.01%
|
| Candles
| 0.02%
|
| Open Lamps (whale oil, kerosene, etc.)
| 0.05%
|
| Lanterns
| 0.05%
|
| Gas Flame Lamps
| 0.05%
|
| Gas Flame Lamps with Mantles
| 0.4%
|
| Carbon Arc Lamps
| 2%
|
| Incandescent Light Bulb
| 5%
|
| Halogen/Quartz Iodide Bulb
| 8%
|
| Fluorescent Tubes (phosphors)
| 35%
|
| Gas Arc Lamps (mercury, sodium,multi-gas, etc.)
| 30% - 60%
|
| Discharge Tubes (neon signs)
| 20% - 40%
|
| LASERS
| 5% to 30%
|
| "Cool" Chemical Light
| 2% to 20%
|
| Light Emitting Diodes (LED's)
| 20% to 40%
|
|


 Fluorescence
Fluorescence