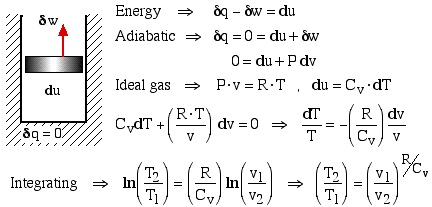
It is unusual to develop the equations of the Adiabatic Process directly from the energy equations, since they are usually introduced after the discussion of entropy as the Isentropic Process. This approach was introduced to us by Potter and Somerton in their Schaum's Outline of Thermodynamics for Engineers, and enables early meaningful analysis of the adiabatic processes in Diesel and Otto cycle engines.
Consider a stationary closed adiabatic system in which the only energy interaction is boundary work, that is, all other work and heat interactions are excluded. The differential form of the energy is thus:
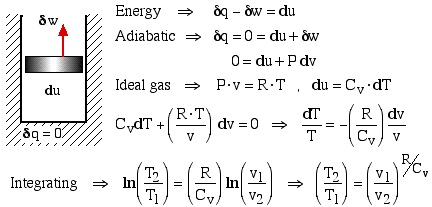
From the web page on Specific Heat Capacity of an Ideal Gas we have:
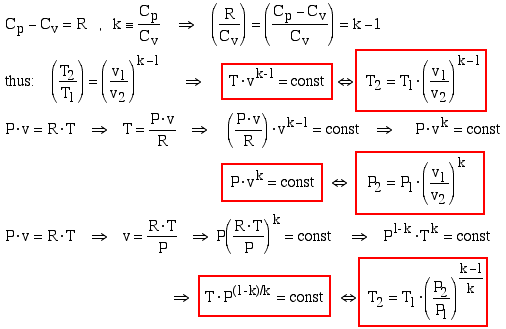 ______________________________________________________________________________________
______________________________________________________________________________________
Digression: The adiabatic process is fundamental to the analysis of water rockets. Refer: Water Rockets - the transportation system of the future (tounge-in-cheek)
______________________________________________________________________________________

Engineering Thermodynamics by
Israel Urieli is licensed under a
Creative Commons Attribution-Noncommercial-Share
Alike 3.0 United States License