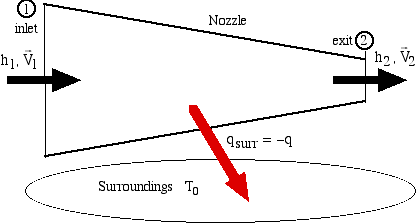
In this example we consider the nozzle of an aircraft jet engine.
One may wonder at the purpose of determining the maximum available work potential of a nozzle, whose entire purpose is to maximise the kinetic energy at the exit state (2). Indeed, a perfect nozzle is isentropic, and has no work potential, since any work done would decrease the kinetic energy. In a practical nozzle there could be an increase in entropy from state (1) to state (2) as well as possible heat loss to the surroundings which will decrease the kinetic energy output and thus the exergy analysis is mainly in order to evaluate this lost kinetic energy potential, or the irreversibility. We recall that kinetic energy is entirely equivalent to exergy.
Recall the previous section in which we determined the irreversibility (irrev) as follows:
Thus referring to the figure above the irreversibility is given by:
However, since there is no actual work done in the nozzle, we have from the energy equation:
Thus by substituting q we obtain the irreversibility as:
Once again, in order to get an intuitive understanding of this analysis we consider the following equivalent nozzle in which we wish to capture the heat loss over the entire nozzle by means of summing the work output of an infinite number of elemental reversible heat engines.
The reason for the many elemental reversible heat engines is that the temperature T of the nozzle varies continuously between the inlet state (1) and the outlet state (2), thus:
However, since there is no actual work done in the nozzle, we have from the energy equation:
Thus by substitution we obtain the final available work potential form:
Since there is no actual work done, the irreversibility can be determined as follows:
Note that this equation is identical to that for the irreversibility given above.
______________________________________________________________________________________
![]()
Engineering Thermodynamics by Israel
Urieli is licensed under a Creative
Commons Attribution-Noncommercial-Share Alike 3.0 United States
License