|
Energy and Radiation
 Digging
Deeper into
Radiation and Selective Absorption Digging
Deeper into
Radiation and Selective Absorption
So why is the atmosphere a selective absorber? The
answer is
found at the atomic level where electrons orbiting the nucleus of an
atom are excited when struck by a bundle of energy. Though we
describe electromagnetic radiation as invisible waves of energy,
at the smallest scale it behaves as a particle, like when light is
emitted by a
single atom or molecule. When energy is given off, there is a change in
the
orbital pattern of the electrons that surround the nucleus of an atom.
As the
orbit changes, a bundle of energy called a "photon"
is
released. Particles of light differ from particles of matter: they have
no mass, occupy no space, and travel at the speed of light, 2.9998 X 108
m s-1. The amount of energy carried by a photon
varies
inversely with
wavelength, the shorter the wavelength, the more energetic the photon.
Electrons orbit the nucleus of an atom at fixed orbital
distances called orbital
shells.
The orbital shell for each atom is different and discrete. That is, for
a given
atom like hydrogen, its electrons can only
orbit at particular distances and are different than those for atoms of
neon.
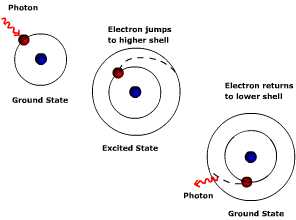 Figure
4-b Effect of photon absorption on electron orbit. Figure
4-b Effect of photon absorption on electron orbit.
Each orbital shell is associated with a given energy
level;
the greater the distance from the nucleus the greater
the energy level. Electrons jump to a higher shell when excited by the
absorption of energy. The photon must have the exact amount of
energy to move the electron from, say, shell one to shell two. If the
photon
doesn't have enough energy to move the electron to shell two, it cannot
move the
electron half way between shell one and two. The atom does not stay in
this excited,
unstable state for very long. Energy is given off and the electron
returns to a
stable state or its "ground state" (lowest energy level or
orbital distance). Recall that the amount of energy carried by a photon
depends
on the wavelength. Thus the atoms that comprise a gas can only absorb,
or
emit, particular wavelengths of energy (i.e. photons of
energy).
Previous | Continue
|
