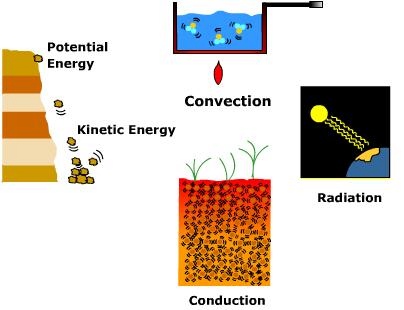
Energy and HeatEnergy is the ability to do work on matter. The work done is manifested in a variety of ways. Matter can be pushed, pulled, or lifted over distance when energy is applied. In other words, work done on matter implies a change of position or movement. Potential energy is the energy of position. A block of rock attached to a high cliff face has substantial potential energy due to its position above the ground. When it breaks away from the cliff and falls to the surface potential energy is converted into kinetic energy of motion. When the rock hits the ground, kinetic energy is converted into work when it dislodges surface material.
Heat or, thermal energy is the total energy associated with random atomic and molecular motions of a substance. Heat is transferred in three ways. Radiation is the transfer of energy via electromagnetic waves. Radiation does not need an intervening medium to pass heat energy from the emitter to the absorber. When radiation from the Sun is absorbed by the Earth it does work by setting molecules in motion and raising their kinetic energy level. In a solid, the molecules may vibrate more rapidly and collide with one another and transfer heat from warmer to colder portions of the mass by conduction. Though conduction is typically thought of occurring within a solid, it can occur between a solid and a fluid. When air, a fluid, comes in contact with the ground, a solid, heat can be transferred through molecular collisions. In fluids like air and water, heat is transferred by the circulation of molecules via the process of convection. Convection implies a vertical transfer of heat, like that which is occurs in a heated pot of water. As water warms it circulates to the surface. The same is true for air. When air is heated by the earth's surface it too circulates upward. While convection is applied to vertical transfer of heat, advection is a term that is applied to the horizontal transfer of heat by the wind. Don't confuse temperature and heat, they are not the same thing. Temperature is a measure of the average kinetic energy level of a substance, in other words, the degree of hotness or coldness. Heat is the total energy associated with the motion of molecules while temperature is the average energy level. A boiling pan of water has a higher temperature than a tepid bathtub of water, but the tub contains more heat because there is more mass. The calorie is a unit of measurement for heat. A calorie is the amount of heat required to raise the temperature of one gram of water through 1oC. Energy is expressed in terms of joules. One joule is the equivalent of one watt of power radiated or dissipated for one second. Specific heat is the heat required to raise the temperature of one unit substance (e.g., gram) through a particular temperature interval (1oC, for example). The specific heat of water is 1 calorie/gram °C = 4.186 joule/gram °C which is higher than any other common substance on Earth. This is one reason why large bodies of water play such an important role in the heat budget of the Earth system. Radiation is often measured in watts per meter2 or langleys per minute. |