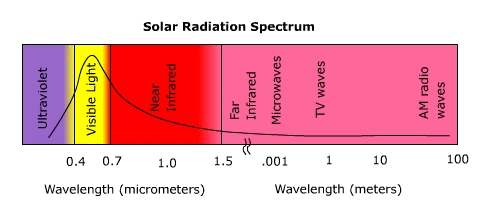
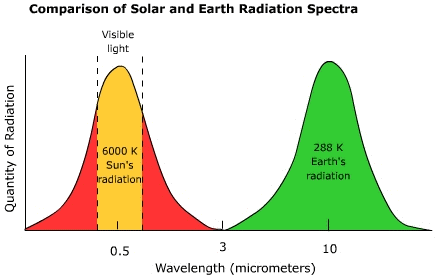
The Nature of Electromagnetic RadiationUnlike convection or conduction, heat transfer by electromagnetic radiation requires no intervening medium to transmit it. Electromagnetic radiation travels through space in the form of waves. It's hard to imagine radiation moving as waves through empty space without a medium to transfer the wave form. The waves created when you drop a rock into a pool require molecules of water to propagate them, but not so for radiation. Energy as electromagnetic wavesThe quantity of energy carried in a wave is associated with the height or amplitude of the wave. Everything else being equal, the amount of energy carried in a wave is directly proportional to the amplitude of the wave. The type or "quality" of radiation depends on the wavelength, the distance between successive crests. The greater the distance between wave crests, the longer the wavelength. Any body that has a temperature is emitting electromagnetic radiation. There are an infinite number of wavelengths that make up the electromagnetic spectrum though we group them into a number of bands (Figure 4.3). The shortest wavelengths fall into the gamma rays, the electromagnetic radiation we can see with our eyes and processed by our brains falls into the visible band, and radio waves are comprised of the longest wavelengths. Figure 4.3 The Solar radiation spectrum The maximum wavelength at which a body emits radiation depends on its temperature. Wein's (pronounced "weens") Law states that the peak wavelength of radiation emission is inversely related to the temperature of the emitting body. That is, the hotter the body, the shorter the wavelength of peak emission. Figure 4.4 shows the wavelengths over which the Sun and Earth emit most of their radiation. The Sun being a much hotter body emits most of its radiation in the shortwave end and the Earth in the longwave end of the spectrum. The division between shortwave and longwave radiation occurs at about 3 micrometers. Figure 4.4 Comparison of solar and earth radiation spectra Radiation as particlesIt's hard to imagine radiation moving as waves through empty space without a medium to transfer the wave form. For instance, the waves created when you drop a rock into a pool of water require molecules of H2O to propagate them. Though we describe electromagnetic radiation as invisible waves of energy, at the smallest scale it behaves as a particle, like when light is emitted by a single atom or molecule. When energy is given off there is a change in the orbital pattern of the electrons that surround the nucleus of an atom. As the orbit changes, a bundle of energy called a "photon" is released. However, particles of light differ from particles of matter: they have no mass, occupy no space, and travel at the speed of light, 2.9998 X 108 m s-1. The amount of energy carried by a photon varies inversely with wavelength, the shorter the wavelength, the more energetic the photon. |