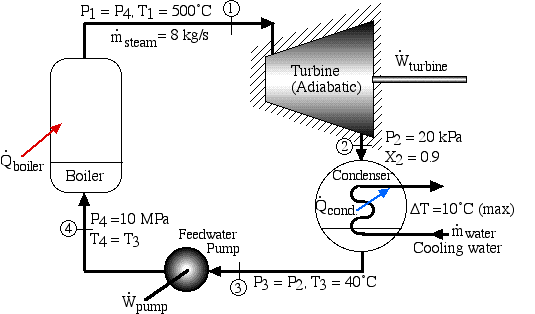
A basic steam power plant consists of four interconnected components, typically as shown in the figure below. These include a steam turbine to produce mechanical shaft power, a condenser which uses external cooling water to condense the steam to liquid water, a feedwater pump to pump the liquid to a high pressure, and a boiler which is externally heated to boil the water to superheated steam. Unless otherwise specified we assume that the turbine and the pump (as well as all the interconnecting tubing) are adiabatic, and that the condenser exchanges all of its heat with the cooling water.
A Simple Steam Power Plant Example - In this example we wish to determine the performance of this basic steam power plant under the conditions shown in the diagram, including the power of the turbine and feedwater pump, heat transfer rates of the boiler and condenser, and thermal efficiency of the system.

In this example we wish to evaluate the following:
Turbine output power and the power required to drive the feedwater pump
Heat power supplied to the boiler and that rejected in the condenser to the cooling water
The thermal efficiency of the power plant (ηth), defined as the net work done by the system divided by the heat supplied to the boiler.
The minimum mass flow rate of the cooling water in the condenser required for a specific temperature rise
Do not be intimidated by the complexity of this system. We will find that we can solve each component of this system separately and independently of all the other components, always using the same approach and the same basic equations. We first use the information given in the above schematic to plot the four processes (1)-(2)-(3)-(4)-(1) on the P-h diagram. Notice that the fluid entering and exiting the boiler is at the high pressure 10 MPa, and similarly that entering and exiting the condenser is at the low pressure 20 kPa. State (1) is given by the intersection of 10 MPa and 500°C, and state (2) is given as 20 kPa at 90% quality, State (3) is given by the intersection of 20 kPa and 40°C, and the feedwater pumping process (3)-(4) follows the constant temperature line, since T4 = T3 = 40°C, .
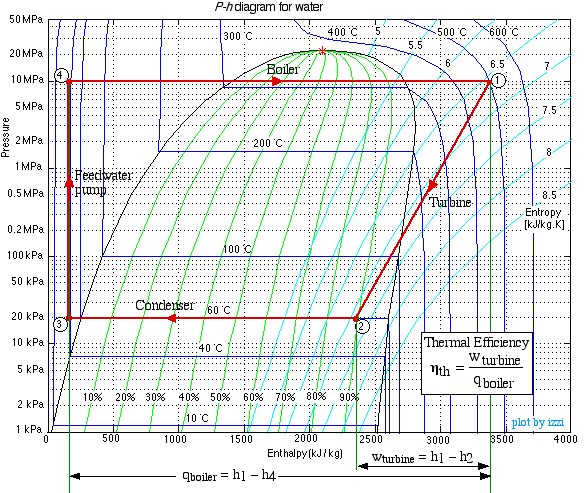
Notice from the P-h diagram plot how we can get an instant visual appreciation of the system performance, in particular the thermal efficiency of the system by comparing the enthalpy difference of the turbine (1)-(2) to that of the boiler (4)-(1). We also notice that the power required by the feedwater pump (3)-(4) is negligible compared to any other component in the system.
(Note: We find it strange that the only thermodynamics text that we know of that even considered the use of the P-h diagram for steam power plants is Engineering Thermodynamics - Jones and Dugan (1995). It is widely used for refrigeration systems, however not for steam power plants.)
We now consider each component as a separate control volume and apply the energy equation, starting with the steam turbine. The steam turbine uses the high-pressure - high-temperature steam at the inlet port (1) to produce shaft power by expanding the steam through the turbine blades, and the resulting low-pressure - low-temperature steam is rejected to the condenser at port (2). Notice that we have assumed that the kinetic and potential energy change of the fluid is negligible, and that the turbine is adiabatic. In fact any heat loss to the surroundings or kinetic energy increase would be at the expense of output power, thus practical systems are designed to minimise these loss effects. The required values of enthalpy for the inlet and outlet ports are determined from the steam tables.
Thus we see that under the conditions shown the steam turbine will produce 8MW of power.
The very low-pressure steam at port (2) is now directed to a condenser in which heat is extracted by cooling water from a nearby river (or a cooling tower) and the steam is condensed into the subcooled liquid region. The analysis of the condenser may require determining the mass flow rate of the cooling water needed to limit the temperature rise to a certain amount - in this example to 10°C. This is shown on the following diagram of the condenser:
Notice that our steam tables do not include the subcooled (or compressed) liquid region that we find at the outlet of the condenser at port (3). In this region we notice from the P-h diagram that over an extremely high pressure range the enthalpy of the liquid is equal to the saturated liquid enthalpy at the same temperature, thus to a close approximation h3 = hf@40°C, independent of the pressure.
Thus we see that under the conditions shown, 17.6 MW of heat is extracted from the steam in the condenser.
I have often been queried by students as to why we have to reject such a large amount of heat in the condenser causing such a large decrease in thermal efficiency of the power plant. Without going into the philosophical aspects of the Second Law (which we cover later in Chapter 5, my best reply was provided to me by Randy Sheidler, a senior engineer at the Gavin Power Plant. He stated that the Fourth Law of Thermodynamics states: "You can't pump steam!", so until we condense all the steam into liquid water by extracting 17.6 MW of heat, we cannot pump it to the high pressure to complete the cycle. (Randy could not give me a reference to the source of this amazing observation).
In order to determine the enthalpy change Δh of the cooling water (or in the feedwater pump which follows), we consider the water to be an Incompressible Liquid, and evaluate Δh as follows:
From the steam tables we find that the specific heat capacity for liquid water CH2O = 4.18 kJ/kg°C. Using this analysis we found on the condenser diagram above that the required mass flow rate of the cooling water is 421 kg/s. If this flow rate cannot be supported by a nearby river then a cooling tower must be included in the power plant design.
We now consider the feedwater pump as follows:
Thus as we suspected from the above P-h diagram plot, the pump power required is extremely low compared to any other component in the system, being only 1% that of the turbine output power produced.
The final component that we consider is the boiler, as follows:
Thus we see that under the conditions shown the heat power required by the boiler is 25.7 MW. This is normally supplied by combustion (or nuclear power). We now have all the information needed to determine the thermal efficiency of the steam power plant as follows:
Note that the feedwater pump work can normally be neglected.
______________________________________________________________________________________
Problem 4.3 - A Geothermal Hybrid Steam Power Plant
Problem 4.4 - Solar Pond Hybrid Steam Power Plant
Problem 4.5 - A Cogeneration Steam Power Plant
Problem 4.6 - An Open Feedwater Heater added to the Cogeneration Steam Power Plant
______________________________________________________________________________________
______________________________________________________________________________________
![]()
Engineering Thermodynamics by Israel
Urieli is licensed under a Creative
Commons Attribution-Noncommercial-Share Alike 3.0 United States
License