|
Plasma membrane-cytoskeleton interactions
A major focus of research in my
laboratory concerns the regulation of interactions
between the plasma membrane and the actin
cytoskeleton, particularly in epithelial cells. The
attachment of membrane proteins to actin filaments
just underneath the membrane is a dynamic process
controlled by a variety of accessory proteins and
certain signal transduction pathways. Actin
filaments organize as parallel bundles or branching
networks. The types of arrangements are fundamental
to the microscopic architecture of cells and tissues
because they influence cell shape, cell-cell and
cell-substrate adhesions, cell division, cell
motility, and cell surface topography. Specifically,
we are interested in understanding molecular
mechanisms required for formation of distinct actin-based
cell surface protrusions such as lamellipodia,
filopodia, microvilli, and sensory stereocilia.
CLIC5: a cytoskeletal protein required for proper
hearing and balance
CLIC5 (chloride intracellular
channel 5) was originally purified from isolated
human placental microvilli, where it was found in a
protein complex containing actin and several actin-binding
proteins. One such protein was Ezrin, a member of
the ERM (Ezrin/Radixin/Moesin) family of membrane-cytoskeletal
crosslinking proteins. Gene mutations that disrupt
expression of CLIC5 cause deafness and vertigo in
both mice and humans. In the jitterbug mouse mutant,
loss of CLIC5 leads to severe morphological defects
on the surface of auditory and vestibular hair cells
in the inner ear. A similar defect occurs in Radixin-deficient
mice. These defects are likely the result of
unstable membrane-cytoskeletal attachments within
the pencil-shaped mechanosensory stereocilia that
project from the apical surface of hair cells. Our
overall hypothesis (see Figure, right) is that CLIC5 and Radixin are part of a protein complex that contains
several other known deafness-associated proteins,
and that this complex is essential to maintain the
structural integrity of stereocilia over the course
of a lifetime.
A CLIC gene in Drosophila, the
fruit fly
Humans and rodents have 6 CLIC-related
genes (CLIC1-6) that have been implicated in a
variety of cellular processes, including ion
transport, signal transduction, cell
differentiation, epithelial tube formation, cell
division, apoptosis, response to cellular stress,
membrane trafficking, and cytoskeletal organization.
Inherent to the study of multi-gene families is the
potential for functional redundancy, which can
complicate interpretation of experiments in
organisms or individual cells expressing multiple
family members. Invertebrate organisms, such as the
fruit fly (Drosophila melanogaster) and worm (Caenorhabditis
elegans), have proven to be extremely powerful
deciphering the functional significance of many
human gene families. In collaboration with
Dr. Soichi Tanda in the Department of Biological
Sciences at Ohio University, we are taking advantage
of Drosophila as a model system to investigate the
biological significance and cellular functions of
CLICs. In addition to the many practical advantages
and its battery of sophisticated genetic tools,
another reason for using Drosophila is that it has
only a single CLIC gene, thereby minimizing
functional redundancies. |
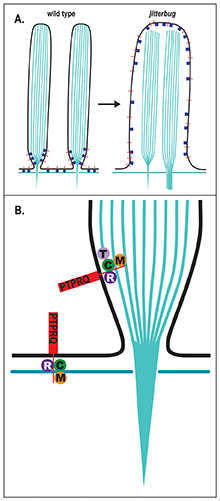 |
Simplified model
for membrane-cytoskeletal linking
complex at the base of hair cell
stereocilia. For details, see Figure
12, Salles et al., 2014
 |
|
|
|
|
. |
|
|
| |
|