|
Funding: NIH-NHGRI-1R15HG009972
|
|
Super Resolution Imaging (Funded by National Human Genome Research Institute of NIH)
|
|
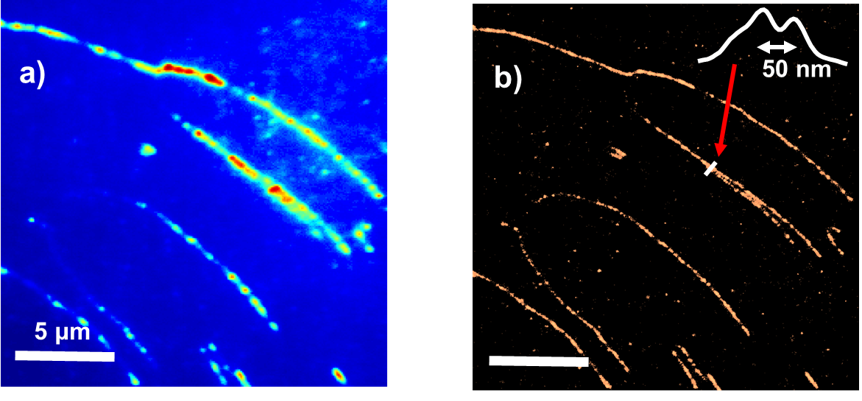
Super-resolution optical microscopy/nanoscopy has greatly extended our understanding of many systems, such as living cell structures and dynamics, and catalytically hot-spots on a nanoparticle. Super-resolution techniques allow the capture of images with a higher resolution than the diffraction limit, ~250 nm for fluorescence images with the highest resolution.
Due to the importance of these techniques to fundamental research, three pioneers in this field has been awarded the 2014 Nobel Prize in Chemistry. We are following these giants in applying the techniques in a various of area such as biophysics and materials science.
|
|
|
The group focuses on applying super-resolution imaging techniques to study molecular dynamics at interfaces. The PI has applied motion blur point accumulation for imaging in nanoscale topography (mbPAINT) to study DNA and protein imaging beyond the diffraction limit of light. The group continues to explore the opportunities in applying this technique in DNA super-resolution optical mapping.
|
| |
|
Photophysics of Various Molecules/Nanomaterials
|
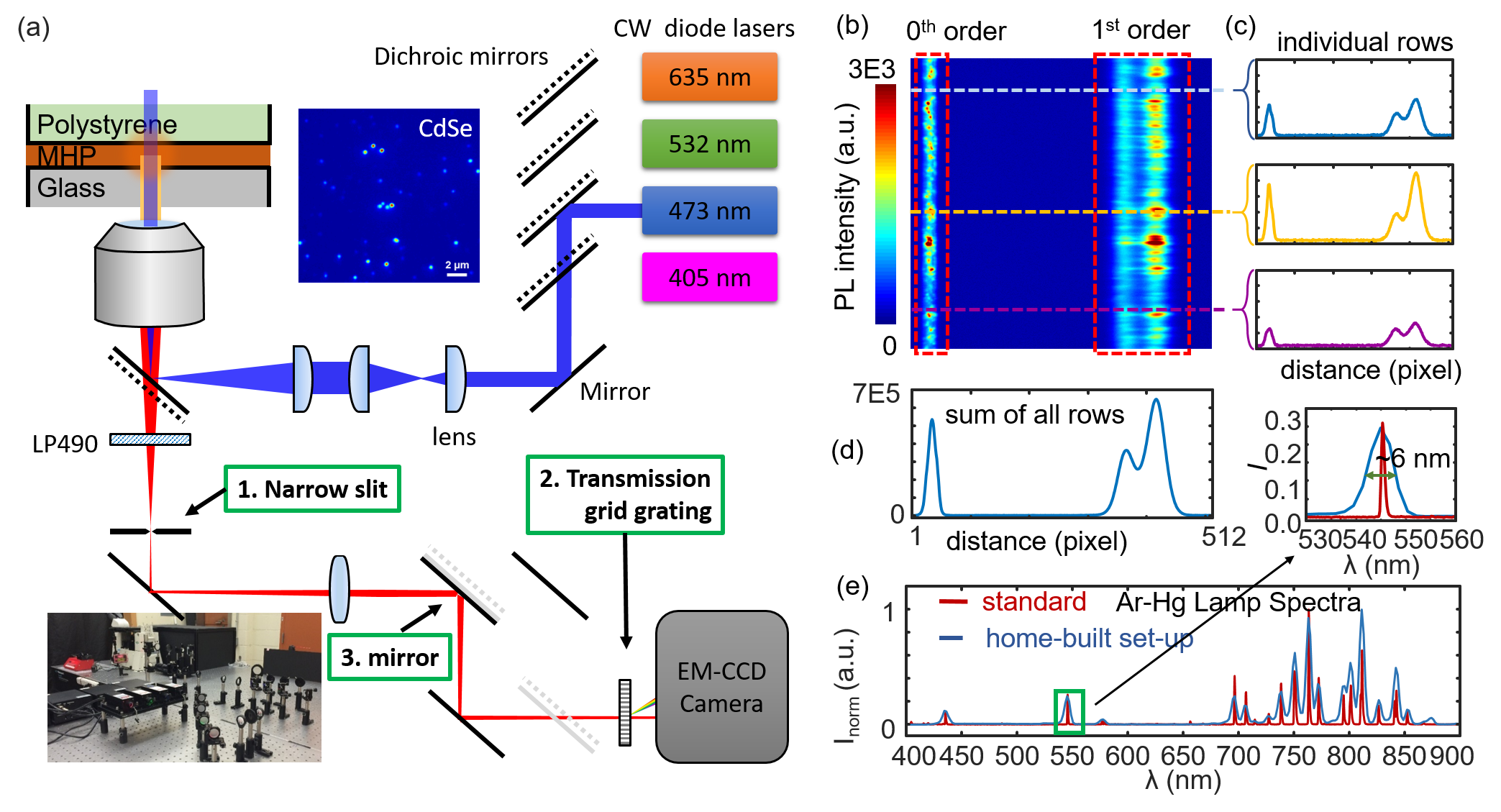 |
| We are developing spectroscopic and microscopic solutions to study the photophysical properties of single molecules, single particles, and bulk thin films. For example, we have added FRET and hyperspectral microscopy functions to our super-resolution microscope and added gas environment and temperature control stages as well. We also collaborate with other groups in ultrafast spectroscopies of organic and inorganic dye systems. The above figure shows some examples of the setup, (a) left showing the scheme of the light path, (b, c, d) right showing measurement of perovskite thin film emmision, and (e) calibration of the spectromicroscopy. |
| |
|
Experiments and Simulations of Single-Molecule Kinetics
|
|
|
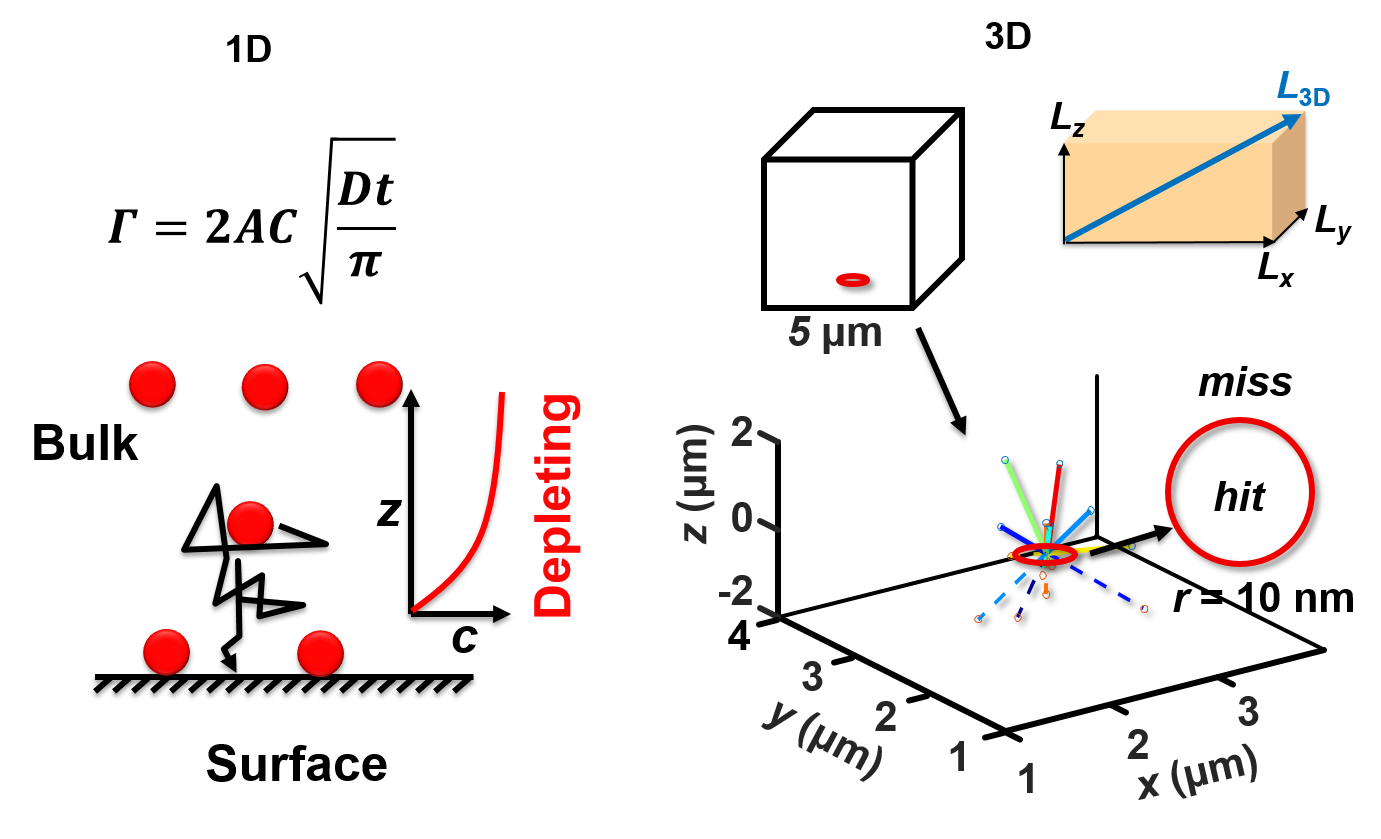
We also synthesize various nanoparticles and quantum dots for photoluminescence measurement and nanodevice fabrication. We use fluorescence microscopy to measure single-molecule diffusion/binding kinetics, single-molecule FRET kinetics, and single-particle fluorescence kinetics. And we use statistical methods to analyze these data. The left figure shows examples of using Monte Carlo simulations for the adsorption of molecules on a surface with 1D and 3D models. We are developing MATLAB codes for data analysis, fitting, and simulations on various kinetic systems, such as smFRET, reaction kinetics, and diffusion. |
|
|
|
Solar Cells and nanofabrication
|
|
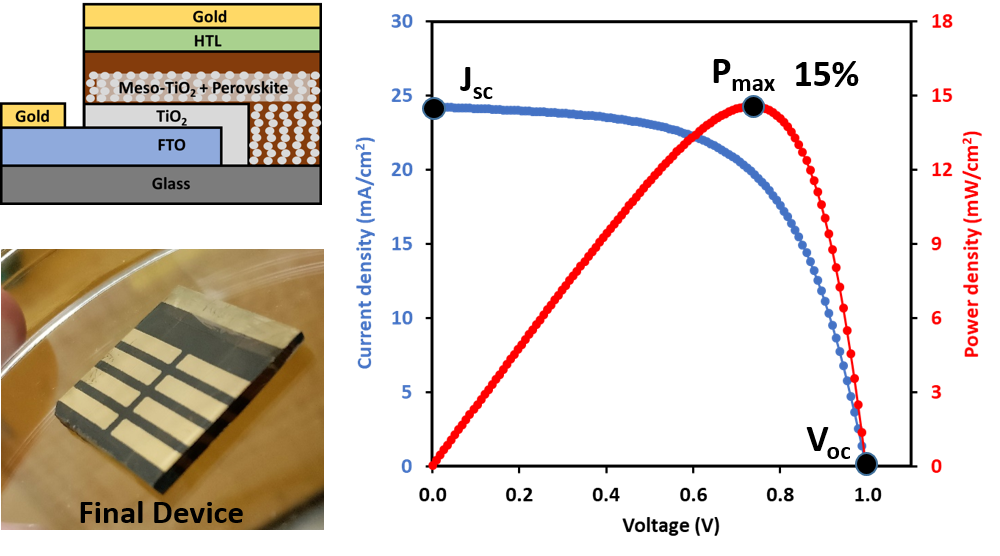
Our group is making thin-film solar cells and characterizing their interfacial chemical and electrochemical properties. The right figure shows a perovskite solar cell (PSC) fabricated and tested in our lab. PSC is a type of promising next generation solar cells.
|
 |
|
|