The Atmosphere
Our atmosphere is a dynamic mixture of gases that envelop the Earth. Two gases, nitrogen and oxygen, make up most of the atmosphere by volume. They are indeed important for maintaining life and driving a number of processes near the surface of the Earth. Many of the so called "minor gases" (known here as "variable gases") play an equally important role in the Earth system. These gases include those that have a significant impact on the heat budget and the availability of moisture across the Earth. The atmosphere is not a homogeneous mass of gases, but has a layered structure as defined by vertical temperature changes.
Atmospheric Composition
Two broad regions can be identified using air composition as a means to subdivide the atmosphere. The heterosphere is the outer most sphere where gases are distributed in distinct layers by gravity according to their atomic weight. Extending from an altitude of 80 km (50 mi), the lightest elements (hydrogen and helium) are found at the outer margins of the atmosphere. The heavier elements (nitrogen and oxygen) are found at the base of the layer.
Figure 3.1 Heterosphere and Homosphere (Not available yet)
The homosphere lies between the Earth's surface and the heterosphere. Gases are nearly uniformly mixed through this layer even though density decreases with height above the surface. The only exceptions is the "ozone layer" from 19 to 50 km (12 to 31 mi) and near surface variations in water vapor, carbon dioxide and air pollutants.
Constant Gases
Nitrogen, oxygen and argon are called the "constant gases" because their concentration has remained virtually the same for much of recent earth history. Nitrogen (78%)is a relatively inert gas produced primarily by volcanic activity. It is an important component of protein in meat, milk, eggs and the tissues of plants, especially grains and members of the pea family. It cannot be ingested directly by organisms but made available to plants, and then to animals, by compounds in the soil. Most atmospheric nitrogen enters the soil by nitrogen-fixing microorganisms.
Oxygen (21%) is important for plant and animal respiratory processes. It is also important to chemical reactions (oxidation) that breakdown rock materials (chemical weathering). Without oxygen, things cannot burn either. Free oxygen in the atmosphere is a product of plant photosynthesis. Plants take up carbon dioxide and in the process of photosynthesis release oxygen.
Argon (.93%) is a colorless, odorless relatively inert gas, the reason it use to electric light bulbs, fluorescent tubes. It is used to form inert atmosphere for arc welding, and growing semiconductor crystals.
Variable Gases
The so called "variable gases" are those present in small and variable amounts. These include carbon dioxide, methane, ozone, water vapor, and particulates among others. Even though they represent a tiny portion of the atmosphere as a whole, they exert a great control over our environment.
Carbon dioxide
Carbon dioxide(CO2) makes up only .036% of the atmosphere by volume. Carbon dioxide is essential to photosynthetic processes of plants. Huge quantities of carbon are stored in plant tissue, deposits of coal, peat, oil, and gas. Carbon dioxide is taken in by plants and during photosynthesis is combined with water and energy to form oxygen and carbohydrates. The stored carbohydrates are used to fuel plant respiration and growth. Carbon is also stored in limestone rocks that have formed by the compaction of carbonate-rich shells of ocean life. Because vegetation takes in so much carbon dioxide, we often refer to plants as a "sink" for it.
Carbon dioxide in the atmosphere varies throughout the year, decreasing slightly during the summer as plants leaf out, and then increases during the winter as plants go dormant and photosynthesis decreases. The zigzag pattern of carbon dioxide measurements taken at Mauna Loa, Hawaii in Figure 3.1 below illustrates this seasonality.
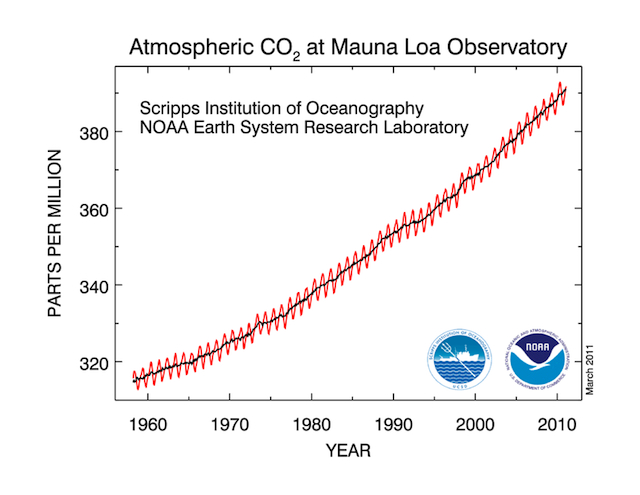
Figure 3.2 Temporal Variation of Carbon Dioxide
Click image to enlarge
Source: NOAA ESRL
The seasonal changes in the geography of uptake and release of carbon dioxide during 2004 is shown in Figure 3.3 by NOAA's CarbonTracker. The black and white dots are locations where CO2 data is collected. A computer model based on this data and a knowledge of surface sources and sinks generates the patterns for the entire globe. Again, the large season to season variation is due to plant life. Blue colors over the northern hemisphere during July in the midlatitudes result from forests and crops soaking up large quantities of CO2. Intense red areas of CO2 release during July, August, and September in the southern hemisphere is largely due to biomass burning. Some burning is natural, such as the dry savanna grasses ignited by lightning strikes. Most is due to people burning fields in preparation for planting, or burning of forests for new agricultural land.
Figure 3.3 NOAA's Carbon Tracker
Courtesy NOAA Earth System Research Laboratory
