|
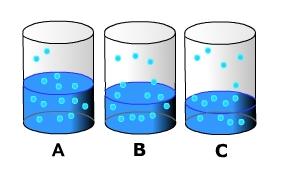
When we think of air as being saturated with moisture we often say that the air is "holding all the moisture it can". This implies that once the air has reached saturation it won't "accept" anymore water by evaporation. This is wrong. So long as there is water available evaporation will continue even when the air is fully saturated. Let's examine the concept of saturation in more detail. Imagine a beaker filled halfway with water. Let's put a top on it to constrain the movement of water molecules and eliminate the influence of wind on evaporation. As the water absorbs heat it begins to change phase and enter the air as water vapor. Above the surface, water vapor molecules dart about suspended in the air. However, near the surface water molecules are attaching themselves back the surface, thus changing back into liquid water (condensation) (A). As evaporation occurs the water level in the beaker decreases (B). This occurs because evaporation exceeds condensation of water back onto the surface. After some time, the amount of water entering the air from evaporation is equal to that condensing (C). When this occurs the air is said to be saturated.
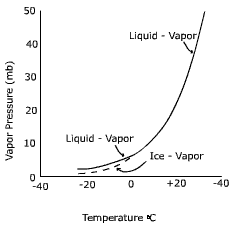
Figure 7.5 Evaporation and condensation The saturation level of the air is directly related to the air's temperature. As air temperature increases, more water can remain in a gas phase. As temperature decreases, water molecules slow down and there is a greater chance for them to condense on to surfaces. The graph below shows the relationship between air temperature and vapor pressure, a measure of the humidity, at saturation.
Note that below zero degrees Celsius the curve splits, one for the saturation point above a liquid surface (liquid-vapor) and one for a surface of ice (ice - vapor). The first thing you might be wondering is how water can exist as a liquid below the freezing point. Water that is not frozen below 0o C is called "super-cooled water". For water to freeze, the molecules must become properly aligned to attach to one another. This is less likely to occur especially with small amounts of water, like cloud droplets. Thus in clouds where temperatures are below freezing it is common to find both super-cooled liquid water and ice crystals. Notice that the saturation vapor pressure at -20o C is lower for ice than for a liquid surface. Why would this be so? You may recall that to convert water from a liquid to a gas requires about 600 calories per gram. To convert water from a solid to a gas requires about 680 calories, hence it is more difficult to "liberate" a molecule of water from ice than water. Therefore, when the air is saturated, there are more molecules above a water surface (i.e. more vapor pressure) than an ice surface (i.e. less vapor pressure). |