Phases of WaterA water molecule is composed of two atoms of hydrogen
and one atom of oxygen. The molecule looks kind of like Mickey Mouse,
with the oxygen forming the head and the two hydrogen forming the ears. Water exists in one of three phases, solid, liquid, or gas. Water molecules cluster together
by bonding to the hydrogen atoms of neighboring water molecules. The molecules that
comprise a solid, like ice, are arranged in a particular order. The molecules do not
circulate but they do move. That is, the molecules vibrate in place around an average
location. Solids represent the lowest level of
kinetic
energy. The molecules of a liquid, like water, have a higher kinetic
energy level
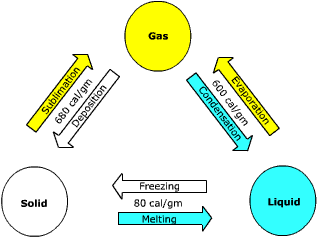
than solids and thus are free to circulate as Figure 7.2 Phase changes of water. In order for water to change from a solid to liquid and finally to a gas, the water molecule must gain energy. The energy absorbed by water is used to break the hydrogen bonds between groups of molecules. When the bonds are broken between the molecules of ice, it melts and they can circulate as a fluid. The energy required to convert ice into water is called the latent heat of fusion. It takes about 80 calories of heat to convert one gram of ice into water. |
 "clumps" of
molecules but constrained by a surface. Molecules of a gas are free to
circulate as well, but are unconfined and move about with the highest kinetic energy level.
"clumps" of
molecules but constrained by a surface. Molecules of a gas are free to
circulate as well, but are unconfined and move about with the highest kinetic energy level.