|
Atmospheric
Moisture
Precipitation processes
The word precipitation in
chemistry refers to material falling out of suspension. The same definition
can be applied when studying weather. Precipitation from a meteorological stand
point is water in some form, falling out of the air, and settling on the surface
of the earth. This allows us to distinguish between forms of condensation in the
atmosphere and condensation that occurs at the surface. Dew is condensation at
the surface and thus is not a form of precipitation. Rain, snow, hail, sleet,
freezing rain are all forms of precipitation. Meteorologists have developed two models of precipitation formation. They are the
collision-coalescence and ice crystal models. An important distinction between the two processes is the
temperature of the cloud. Warm clouds are ones whose mass lies above the
freezing level while cold clouds primarily exist where the
temperature is below freezing (Figure 7.28).
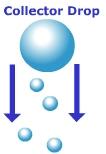
Figure 7.29 Collision-coalescence
of raindrops
The collision-coalescence
model applies to warm clouds that form in the tropics. Warm clouds are those
that form at altitudes where the air temperature is above freezing. For
precipitation to form under this model, there needs to be a variety of different
size condensation nuclei. Large condensation nuclei will create large water
droplets while smaller condensation nuclei create small ones. In order for the
droplets to make their way to the surface they have to be heavy enough to overcome the resistance imposed by upwardly rising air that is fueling the
development of the cloud. The smaller, lighter droplets are easily suspended in
the updrafts of air, while the larger heavy collector droplets fall and collide with
the smaller ones. Upon collision, the droplets coalesce into a bigger droplet.
As the droplet falls, resistance by the air flattens the droplet to the point
where it becomes unstable and breaks apart. With enough collisions, the droplet achieves a size sufficient to fall all the
way to the surface.
The ice-crystal model, or
Bergeron process, is the process of precipitation formation in the middle and high
latitudes. Here, clouds form at altitudes where the temperatures are below the
freezing point of water. In these clouds, water exists in its liquid form even
though the temperatures are cold enough to freeze water. Water that has a
temperature below freezing but is still in a liquid state is called "super-cooled
water". Water in extremely small amounts such as cloud droplets can
exist in such a
state. Ice crystals are found co-existing with the super-cooled water in cold
clouds. When this occurs, the ice crystals will grow at the expense of the water
droplets. Why? Examine the saturation curve in Figure 7.29. It shows that at
temperatures below freezing the saturation vapor pressure of ice is less than
that over a droplet of water. This means that a water vapor gradient exists between
the droplet and the ice. Water can evaporate off the droplet and deposit on the
ice in response to the water vapor gradient. The droplet will dissipate in size while
the ice crystal grows into a snow flake. Once the snow flake is large enough, it
will fall to the surface. Thus, precipitation that falls in the middle and high
latitudes starts out as snow. Whether it hits the surface as snow or rain
depends on the temperature conditions through which the snowflake falls.
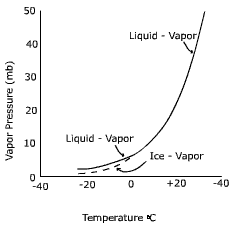
Figure 7. 30 Relationship between air temperature and vapor pressure at
saturation
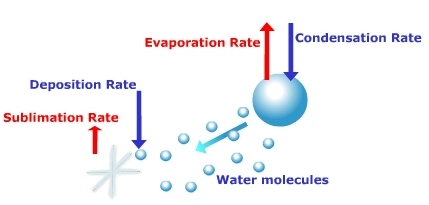
Figure 7.31
Growth of ice crystals by deposition
Previous |
Continue
|