
Following on the Second Law developed in Chapter 5 we consider the Clausius Inequality which leads to the definition of a new property Entropy (S - kJ / K) as follows:
A very strange definition indeed, and difficult to comprehend. It is defined in differential format as the reversible heat transfer divided by the temperature. In an attempt to try and understand it we rewrite the definition as follows:
It is advantageous to compare this definition with the equivalent definition of work as follows:
Thus it begins to make sense. Work done requires both a driving force (pressure P) and movement (volume change dV). We implicitly evaluated the work done for reversible processes - always neglecting friction or any other irreversibility. Similarly we can state that heat transfer requires both a driving force (temperature T) and some equivalent form of "movement" (entropy change dS). Since temperature can be considered as represented by the vibration of the molecules, it is this transfer of vibrational energy that we define as entropy.
We now continue with the Increase in Entropy Principle which is also derived from the Clausius Inequality, and states that for any process, the total change in entropy of a system or control volume together with its enclosing adiabatic surroundings is always greater than or equal to zero. This total change of entropy is denoted the Entropy Generated during the process (Sgen [kJ/K] or sgen [kJ/kg.K]. For reversible processes the entropy generated will always be zero.
We use the differential form of the energy equation to derive the T.ds relations which can be used to evaluate the change of entropy (Δs) for processes involving 2-phase fluids (Steam, R134a, CO2), solids or liquids, or ideal gasses.
Finally we present a convenient Entropy Equation Summary Sheet which summarises the relevant relations concerning entropy generation and evaluation of entropy change Δs. The Isentropic Processes Summary Sheet extends the relations of entropy change to enable the evaluation of isentropic processes.
One of the important applications of isentropic processes is in determining the efficiency of various adiabatic components. These include turbines, compressors and aircraft jet nozzles. Thus we have made the statement that steam turbines are designed to be adiabatic, and that any heat loss from the turbine will result in a reduction in output power, however only now can we make the statement that the ideal turbine is isentropic. This enables us to evaluate the Adiabatic Efficiency (sometimes referred to as isentropic efficiency) of these components, and we extend the isentropic process sheet with an Adiabatic Efficiency Summary Sheet.
There are two property diagrams involving entropy in common usage, the temperature-entropy (T-s) and enthalpy-entropy (h-s) "Mollier" diagrams. We will find that the h-s diagram is extremely useful for evaluating adiabatic turbines and compressors, and complements the P-h diagram which we used in Chapter 4 to evaluate entire steam power plants or refrigerator systems. The h-s diagram for steam is presented below:
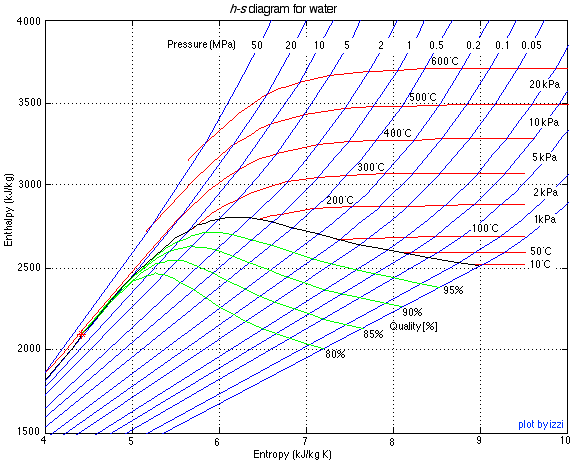
The important characteristic of the h_s diagram is that the ideal adiabatic turbine can be conveniently plotted as a vertical line, allowing an intuitive visual appreciation of the turbine performance. We define the turbine adiabatic efficiency as follows:
Notice that for the for the actual turbine there will always be an increase in entropy, which means that the turbine adiabatic efficiency will always be less than 100%.
An Adiabatic Steam Turbine Example
Consider an adiabatic steam turbine having a turbine adiabatic efficiency ηT = 80%, operating under the conditions shown in the following diagram:
a) Using steam tables, determine the enthalpy and entropy values at station (1) and station (2s) assuming that the turbine is isentropic. [h1 = 3479 kJ/kg, s1 = 7.764 kJ/kg.K; h2s = 2461 kJ/kg, s2s = s1]
b) From the definition of turbine adiabatic efficiency (shown on the diagram), and given that ηT = 80%, determine the actual enthalpy and entropy values as well as the temperature at station (2a). [h2a = 2665 kJ/kg, s2a = 8.38 kJ/kg.K, T2a = 88°C]
c) Plot the actual and isentropic turbine processes (Stations (1)-(2a) and (1)-(2s)) on the enthalpy-entropy h-s "Mollier" diagram, and indicate the actual turbine specific work (wa) as well as the isentropic turbine specific work (ws) on the diagram.
d) Determine the actual power output of the turbine (kW). [1629 kW]
The h-s diagram plot follows. Notice that we have indicated all the enthalpy and entropy values (which we determined from the steam tables) on the plot. This allows a check on the feasibility of our results.

We take a somewhat simpler approach for incompressible liquids, as in the following problem.
Unfortunately we find that at supercritical pressures and temperatures > 100°C, liquid water no longer behaves as an incompressible liquid, as in the following:
Refrigerant R134a Compressors
One of the interesting aspects of compressors is that they can be made more efficient by cooling. The reason why we still consider the adiabatic efficiency of compressors that are normally found in refrigeration, air-condition and heat pump systems is that it is considered to be impractical to cool them. Thus the ideal compressor (absorbing a minimum of power) is considered to be isentropic, and we define compressor adiabatic efficiency as follows:
We have also provided an R134a enthalpy-entropy (h-s) diagram which we find useful for evaluating adiabatic compressors that are normally found in refrigeration, air-condition and heat pump systems.
Solved Problem 6.5 - Adiabatic Efficiency of a R134a Compressor
Problem 6.7 - Recall in Chapter 4c that we provided Problem 4.7 concerning a home refrigerator, and examining it's performance before and after adding an internal heat exchanger.
a) Plot the actual and the isentropic compressor processes on the enthalpy-entropy (h-s) diagram provided, for both cases - with and without the internal heat exchanger.
b) Using the R134a tables determine the actual compressor adiabatic efficiency (ηC) for both cases [75%, 76%]
Problem 6.8 - Recall in Chapter 4c that we provided Problem 4.9 concerning an innovative home air conditioner and hot water heating system, in which we determined the COP for both the air conditioning and the water heating systems. Do sections a) and b) specified in Problem 6.7 above on the compressor of this home air conditioning system. [ηC = 76%]
Problem 6.9 - Adiabatic Efficiency of a High Pressure R134a Compressor
Problem 6.10 - Adiabatic Efficiency of a CO2 (R744) Compressor
We will also extend the h-s diagram into the ideal gas region and use it to advantage when we consider gas turbine and jet engine systems in Part b) that follows.
______________________________________________________________________________________
![]()
Engineering Thermodynamics by Israel
Urieli is licensed under a Creative
Commons Attribution-Noncommercial-Share Alike 3.0 United States
License