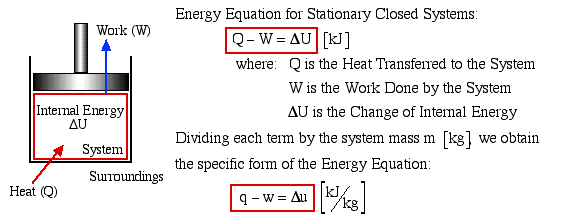
We consider the First Law of Thermodynamics applied to stationary closed systems as a conservation of energy principle. Thus energy is transferred between the system and the surroundings in the form of heat and work, resulting in a change of internal energy of the system. Internal energy change can be considered as a measure of molecular activity associated with change of phase or temperature of the system and the energy equation is represented as follows:
Energy transferred across the boundary of a system in the form of heat always results from a difference in temperature between the system and its immediate surroundings. We will not consider the mode of heat transfer, whether by conduction, convection or radiation, thus the quantity of heat transferred during any process will either be specified or evaluated as the unknown of the energy equation. By convention, positive heat is that transferred from the surroundings to the system, resulting in an increase in internal energy of the system
In this course we consider three modes of work transfer across the boundary of a system, as shown in the following diagram:
In this course we are primarily concerned with Boundary Work due to compression or expansion of a system in a piston-cylinder device as shown above. In all cases we assume a perfect seal (no mass flow in or out of the system), no loss due to friction, and quasi-equilibrium processes in that for each incremental movement of the piston equilibrium conditions are maintained. By convention positive work is that done by the system on the surroundings, and negative work is that done by the surroundings on the system, Thus since negative work results in an increase in internal energy of the system, this explains the negative sign in the above energy equation.
Boundary work is evaluated by integrating the force F multiplied by the incremental distance moved dx between an initial state (1) to a final state (2). We normally deal with a piston-cylinder device, thus the force can be replaced by the piston area A multiplied by the pressure P, allowing us to replace A.dx by the change in volume dV, as follows:
This is shown in the following schematic diagram, where we recall that integration can be represented by the area under the curve.
Note that work done is a Path Function and not a property, thus it is dependent on the process path between the initial and final states. Recall in Chapter 1 that we introduced some typical process paths of interest:
Isothermal (constant temperature process)
Isochoric or Isometric (constant volume process)
Isobaric (constant pressure process)
Adiabatic (no heat flow to or from the system during the process)
It is sometimes convenient to evaluate the specific work done which can be represented by a P-v diagram thus if the mass of the system is m [kg] we have finally:
We note that work done by the system on the surroundings (expansion process) is positive, and that done on the system by the surroundings (compression process) is negative.
Finally for a closed system Shaft Work (due to a paddle wheel) and Electrical Work (due to a voltage applied to an electrical resistor or motor driving a paddle wheel) will always be negative (work done on the system). Positive forms of shaft work, such as that due to a turbine, will be considered in Chapter 4 when we discuss open systems.
The third component of our Closed System Energy Equation is the change of internal energy resulting from the transfer of heat or work. Since specific internal energy is a property of the system, it is usually presented in the Property Tables such as in the Steam Tables. Consider for example the following solved problem.
Solved Problem 3.1 - Recall the Solved Problem 2.2 in Chapter 2a in which we presented a constant pressure process. We wish to extend the problem to include the energy interactions of the process, hence we restate it as follows:
Two kilograms of water at 25°C are placed in a piston cylinder device under 3.2 MPa pressure as shown in the diagram (State (1)). Heat is added to the water at constant pressure until the temperature of the steam reaches 350°C (State (2)). Determine the work done by the fluid (W) and heat transferred to the fluid (Q) during this process.
Solution Approach:
We first draw the diagram of the process including all the relevant data as follows:
Notice the four questions to the right of the diagram, which we should always ask before attempting to solve any thermodynamic problem. What are we dealing with - liquid? pure fluid, such as steam or refrigerant? ideal gas? In this case it is steam, thus we will use the steam tables to determine the various properties at the various states. Is the mass or volume given? If so we will specify and evaluate the energy equation in kiloJoules rather than specific quantities (kJ/kg). What about entropy? Not so fast - we have not yet considered enthalpy (below) - wait patiently until Chapter 6.
Since work involves the integral of P.dv we find it convenient to sketch the P-v diagram of the problem as follows:
Notice on the P-v diagram how we determine the specific work done as the area under the process curve. We also notice that in the Compressed Liquid region the constant temperature line is essentially vertical. Thus all the property values at State (1) (compressed liquid at 25°C) can be determined from the saturated liquid table values at 25°C.
Enthalpy (h) - a New Property
In the case studies that follow we find that one of the major applications of the closed system energy equation is in heat engine processes in which the system is approximated by an ideal gas, thus we will develop relations to determine the internal energy for an ideal gas. We will find also that a new property called Enthalpy will be useful both for Closed Systems and in particular for Open Systems, such as the components of steam power plants or refrigeration systems. Enthalpy is not a fundamental property, however is a combination of properties and is defined as follows:
As an example of its usage in closed systems, consider the following constant pressure process:
Applying the energy equation we obtain:
![]()
However, since the pressure is constant throughout the process:
Substituting in the energy equation and simplifying:
Values for specific internal energy (u) and specific enthalpy (h) are available from the Steam Tables, however for ideal gasses it is necessary to develop equations for Δu and Δh in terms of Specific Heat Capacities. We develop these equations in terms of the differential form of the energy equation in the following web page:
Specific Heat Capacities of an Ideal Gas
We have provided property values for various ideal gases, including the gas constant and specific heat capacities in the following web page:
Properties of Various Ideal Gases (at 300 K)
__________________________________________________________________
______________________________________________________________________________________
![]()
Engineering Thermodynamics by Israel
Urieli is licensed under a Creative
Commons Attribution-Noncommercial-Share Alike 3.0 United States
License